Abstract
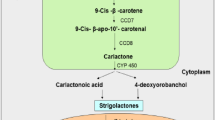
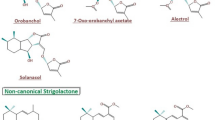
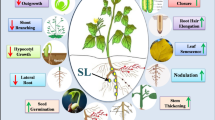
Strigolactones (SLs) are a small class of diverse metabolites derived from the carotenoid pathway. These active biomolecules are a recent inclusion to the list of non-traditional phytohormones or plant growth regulators. Previous reports and articles have discussed their pro-regulatory roles in plant growth, development, signaling and delay of senescence. However, the multi-level control of SL biosynthesis is less known. The anabolic genes are strictly regulated through synchronized co-operation between crucial phytohormones. Epigenetic and microRNA-mediated post-transcriptional regulation fine tunes the cellular accumulation of these putative phytohormones. The question now arises that why such multi-level intricate regulation at all is required for SLs, which were originally detected as under-rated germination and rhizosphere stimulants. This review answers the question in the backdrop of the positive roles of SLs in promoting abiotic stress resilience across diverse plant species. SLs reportedly accumulate in the plant tissues in response to environmental sub-optimal conditions like drought, salinity, temperature, nutrient deprivation and oxidative stresses. Fluctuations in the light quality and intensity also trigger variable accumulation of SLs, indicating their potential in regulating light stress as well. Though the exact roles of SLs have not yet been characterized, it is predicted that they possibly induce the expression of downstream osmolytes to maintain metabolic homeostasis in the stressed cells. Thus, exogenous treatments or transgenic approaches for higher SL bioaccumulation can be potential strategies for developing multiple abiotic stress tolerance in crops and plants.


Similar content being viewed by others
References
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P et al (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Aroca R, Ruiz-Lozano JM, Zamarreno AM, Paz JA, Garcia-Mina JM et al (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Banerjee A, Roychoudhury A (2016) Plant responses to light stress: oxidative damages, photoprotection and role of phytohormones. In: Ahammed GJ, Yu J-Q (eds) Plant hormones under challenging environmental factors. Springer, Dordrecht, pp 181–213
Banerjee A, Roychoudhury A (2017) Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254:3–16
Banerjee A, Roychoudhury A (2018a) The gymnastics of epigenomics in rice. Plant Cell Rep 37:25–49
Banerjee A, Roychoudhury A (2018b) Abiotic stress, generation of reactive oxygen species, and their consequences: an overview. In: Singh VP, Singh S, Tripathi D, Mohan Prasad S, Chauhan DK (eds) Revisiting the role of reactive oxygen species (ROS) in plants: ROS Boon or bane for plants?. Wiley, pp 23–50
Banerjee A, Roychoudhury A, Krishnamoorthi S (2016) Emerging techniques to decipher microRNAs (miRNAs) and their regulatory role in conferring abiotic stress tolerance in plants. Plant Biotechnol Rep 10:185–205
Banerjee A, Wani SH, Roychoudhury A (2017) Epigenetic control of plant cold responses. Front Plant Sci 8:1643
Beveridge CA, Kyozuka J (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13:34–39
Bonneau L, Huguet S, Wipf D, Pauly N, Truong HN (2013) Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favourable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol 199:188–202
Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P et al (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8:443–449
Boyer FD, de Saint Germain A, Pillot JP, Pouvreau JB, Chen VX, Ramos S et al (2012) Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: molecule design for shoot branching. Plant Physiol 159:1524–1544
Braun N, de Saint Germain A, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I et al (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158:225–238
Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150:482–493
Bu Q, Lv T, Shen H, Luong P, Wang J, Wang Z et al (2014) Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol 164:424–439
Cardoso C, Zhang Y, Jamil M, Hepworth J, Charnikhova T, Dimkpa SO et al (2014) Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1orthologs. Proc Natl Acad Sci USA 111:2379–2384
Chen Z, Gao X, Zhang J (2015) Alteration of osa-miR156e expression affects rice plant architecture and strigolactones (SLs) pathway. Plant Cell Rep 34:767–871
Cheng X, Ruyter-Spira C, Bouwmeester H (2013) The interaction between strigolactones and other plant hormones in the regulation of plant development. Front Plant Sci 4:199
Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P et al (1972) Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witch weed (Striga lutea). J Am Chem Soc 94:6198–6199
Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158:487–498
Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152:1914–1927
Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB (2013) Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol Plant 6:76–87
Foo E, Ferguson BJ, Reid JB (2014) The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Ann Bot 113:1037–1045
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Ha CV, Leyva-Gonzalez MA, Osakabe Y, Tran UT, Nishiyama R et al (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA 111:851–856
Hayward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Islam S, Griffiths CA, Blomstedt CK, Le T-N, Gaff DF et al (2013) Increased biomass, seed yield and stress tolerance is conferred in Arabidopsis by a novel enzyme from the resurrection grass Sporobolus stapfianus that glycosylates the strigolactone analogue GR24. PLoS One 8:e80035
Koltai H (2011) Strigolactones are regulators of root development. New Phytol 190:545–549
Koltai H, Cohen M, Chesin O, Mayzlish-Gati E, Bécard G, Puech V et al (2011) Light is a positive regulator of strigolactone levels in tomato roots. J Plant Physiol 168:1993–1996
Lechat MM, Pouvreau JB, Péron T, Gauthier M, Montiel G et al (2012) PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J Exp Bot 63:5311–5322
Lechat MM, Brun G, Montiel G, Véronési C, Simier P, Thoiron S et al (2015) Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in obligate root parasitic plant, Phelipanche ramosa L. Pomel. J Exp Bot 66:3129–3140
Leyser O (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32:694–703
Li W, Nguyen KH, Watanabe Y, Yamaguchi S, Tran LS (2016) OaMAX2 of Orobanche aegyptiaca and Arabidopsis AtMAX2 share conserved functions in both development and drought responses. Biochem Biophys Res Commun 478:521–526
Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318:1302–1305
Liu J, Maldonado-Mendoza I, Lepez-Meyer M, Cheung F, Town CD, Harrison MJ (2007) Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J 50:529–544
Liu W, Kohlen W, Lillo A, den Camp RO, Ivanov S et al (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23:3853–3865
Liu J, Novero M, Charnikhova T, Ferrandino A, Schubert A et al (2013) CAROTENOID CLEAVAGE DIOXYGENASE 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model Lotus japonicus. J Exp Bot 64:1967–1981
Liu J, He H, Vitali M, Visentin I, Charnikhova T et al (2015) Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241:1435–1451
Lopez-Raez JA (2016) How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243:1375–1385
Lopez-Raez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK et al (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytol 187:343–354
Luo L, Li W, Miura K, Ashikari M, Kyozuka J (2012) Control of tiller growth of rice by OsSPL14 and strigolactones, which work in two independent pathways. Plant Cell Physiol 53:1793–1801
Lv S, Zhang Y, Li C, Liu Z, Yang N et al (2017) Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. https://doi.org/10.1111/nph.14813
Makhzoum A, Yousefzadi M, Malik S, Gantet P, Tremouillaux-Guiller J (2017) Strigolactone biology: genes, functional genomics, epigenetics and applications. Crit Rev Biotechnol 37:151–162
Marzec M, Muszynska A (2015) In silico analysis of the genes encoding proteins that are involved in the biosynthesis of the RMS/MAX/D pathway revealed new roles of strigolactones in plants. Int J Mol Sci 16:6757–6782
Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano M et al (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem 73:2460–2465
Matusova R, Rani K, Verstappen FW, Franssen MC, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934
Mayzlish-Gati E, De-Cuyper C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB et al (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341
Mishra S, Upadhyay S, Shukla RK (2017) The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front Physiol 7:691
Ouyang X, Li J, Li G, Li B, Chen B, Shen H et al (2011) Genome-wide binding site analysis of FAR-RED ELONGATED HYPOCOTYL 3 reveals its novel function in Arabidopsis development. Plant Cell 23:2514–2535
Pandey A, Sharma M, Pandey GK (2016) Emerging roles of strigolactones in plant responses to stress and development. Front Plant Sci 7:434
Pelaez-Vico MA, Bernabeu-Roda L, Kohlen W, Soto MJ, Lopez-Raez JA (2016) Strigolactones in the Rhizobium-legume symbiosis: stimulatory effect on bacterial surface motility and down-regulation of their levels in nodulated plants. Plant Sci 245:119–127
Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J et al (2012) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158:1976–1987
Roychoudhury A, Banerjee A (2017) Abscisic acid signaling and involvement of mitogen activated protein kinases and calcium-dependent protein kinases during plant abiotic stress. In: Pandey G (ed) Mechanism of plant hormone signaling under stress, vol 1. Wiley, pp 197–241
Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R (2012) Regulation of arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 11:4033–4044
Ruiz-Lozano JM, Aroca R, Zamarreno AM, Molina S, Andreo-Jimenez B et al (2016) Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ 39:441–452
Saeed W, Naseem S, Ali Z (2017) Strigolactones biosynthesis and their role in abiotic stress resilience in plants: a critical review. Front Plant Sci 8:1487
Saia S, Amato G, Frenda AS, Giambalvo D, Ruisi P (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9:e90738
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 1:e1001474
Smith SM, Li J (2014) Signalling and responses to strigolactones and karrikins. Curr Opin Plant Biol 21:23–29
Stirnberg P, van de Sande K, Leyser HMO (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129:1131–1141
Sun H, Tao J, Liu S, Huang S, Chen S, Xie X et al (2014) Strigolactones are involved in phosphate-and nitrate-deficiency-induced root development and auxin transport in rice. J Exp Bot 65:6735–6746
Trevisan S, Manoli A, Ravazzolo L, Botton A, Pivato M, Quaggiotti S (2015) Nitrate sensing by the maize root apex transition zone: a merged transcriptomic and proteomic survey. J Exp Bot 66:3699–3715
Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y et al (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6:741–749
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126
Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T et al (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J 61:300–311
Wang YH, Irving HR (2011) Developing a model of plant hormone interactions. Plant Signal Behav 6:494–520
Waters MT, Gutjahr C, Bennett T, Nelson DC (2017) Strigolactone signaling and evolution. Annu Rev Plant Biol 68:291–322
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Ann Rev Phytopathol 48:93–117
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240:399–408
Yin R, Skvortsova MY, Loubery S, Ulm R (2016) COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc Natl Acad Sci USA 113:E4415–E4422
Acknowledgements
Financial assistance from Council of Scientific and Industrial Research (CSIR), Government of India, through the research Grant [38(1387)/14/EMR-II] to Dr. Aryadeep Roychoudhury is gratefully acknowledged. The authors are also thankful to University Grants Commission, Government of India, for providing fellowship to Mr. Aditya Banerjee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Huang.
Rights and permissions
About this article
Cite this article
Banerjee, A., Roychoudhury, A. Strigolactones: multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol Plant 40, 86 (2018). https://doi.org/10.1007/s11738-018-2660-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2660-5