Abstract
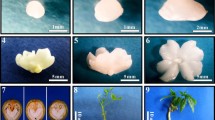
Proteins and activities of antioxidant enzymes, including polyphenol oxidase (PPO), peroxidase (POX) and catalase (CAT), were evaluated in Dendrobium hookerianum during different stages of development of both protocorms and protocorm-like bodies (PLB) derived from seeds and axillary buds, respectively. The changes in the protein contents and the enzyme activities along with their isozyme patterns were compared between these two culture systems. It was found that the protein contents and the enzyme activities increased steadily over time during different stages of development in both the systems. Protein contents (4.57 mg/g fresh wt.) and activities of POX (21.9 U/mg protein), CAT (9.86 U/mg protein) were observed to be higher in PLB system as compared to protocorm system at stage IV of development. However, although the PPO activity increased gradually till the third stage of development, a decline was observed at stage IV wherein the activity was recorded to be more or less same in both the systems. Also, few proteins (~61, 50, 46, 32, 25, 16, 6 kDa) and new isozymes (POX 7, 8; CAT 2) were expressed only in PLB system of development. In general, high protein content and enzyme activities were detected in PLB system as compared to protocorm system. The results of the present study indicate that few proteins and isozymes could be regarded as specific biochemical markers for different stages of development of this medicinally important orchid.



Similar content being viewed by others
References
Aebi H (1984) Catalases. In: Packer L (Ed) Methods in enzymology. Academic Press, Orlando, 105:121–126
Alscher RG, Donahue J, Cramer CL (1997) Reactive oxygen species and antioxidant: relationships in green cells. Physiol Plant 100:224–233
Amako A, Chem K, Asada K (1999) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozyme of ascorbate peroxidase in plants. Plant Cell Physiol 32:497–504
Arditti J (1979) Aspects of the physiology of orchids. In: Woolhouse HW (ed) Advances in botanical research. Academic Press, New York, pp 421–655
Bagnoli F, Capuana M, Racchi ML (1998) Developmental changes of catalase and superoxide dismutase in zygotic and somatic embryos of horse chestnut. Aust J Plant Physiol 25:909–913
Bailey LF, McHargue JS (1943) Enzyme activityin tomato fruits and leaves at different stages of development. Am J Bot 30:763–766
Bailly C, Bogatek-Leszczynska R, Come D, Corbineau F (2002) Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci Res 12:47–55
Bailly C, Leymarie J, Lehner A, Rousseau Come D, Corbineau F (2004) Catalase activity and expression in developing sunflower seeds as related to drying. J Exp Bot 55:475–483
Baťková P, Pospíšilová J, Synková H (2008) Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol Plant 52:413–422
Begum AA, Tamaki M, Tahara M, Kato S (1994) Somatic embryogenesis in Cymbidium through in vitro culture of inner tissue of protocormlike bodies. J Jpn Soc Hortic Sci 63:419–427
Benson EE (2000) Do free radicals have a role in plant tissue culture recalcitrance? In vitro cell Dev Biol Plant 36:163–170
Blackeman JP, Mokahel MA, Hadley G (1976) Effect of mycorrhizal infection on respiration and activity of some oxidase enzyme of orchid protocorms. New Phytol 77:697–704
Bogdanović J, Radotić K, Mitrović A (2008) Changes in activities of antioxidant enzymes during Chenopodium murale seed germination. Biol Plant 52:396–400
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye banding. Anal Biochem 72:248–254
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tissue Org Cult 64:145–157
Chang C, Chang WC (1998) Plant regeneration from callus culture of Cymbidium ensifolium var. misericors. Plant Cell Rep 17:251–255
Chen JT, Chang WC (2000) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 160:87–93
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–787
Chen L, Luthe D (1987) Analysis of proteins from embryogenic and non-embryogenic rice (Oryza sativa, L.) calli. Plant Sci 48:181–188
Chen J, Ziv MN (2001) The effect of ancymidider on hyperhydricity, regeneration, starch and antioxidant enzymatic activities in liquid-cultured Narcissus. Plant Cell Rep 20:22–27
Cortelazzo AL, Marais M, Joseleau J (1996) Changes in peroxidases in the suspension culture of Rubus fruticosus during growth. Plant Cell Tissue Org Cult 46:27–33
Coulson CB, Sim AK (1965) Proteins of wheat flour. Nature 208:583–584
Creemers-Molenaar J, Van Oort Y (1990) Antioxidants increase the plating efficiency and microcallus growth of protoplasts in Lolium perenne L. In: Nijkamp HJJ, Van der plas LHW, Van Aartrijk J (eds) Progress in plant molecular biology. Kluwer Academic publishers, Dordrecht, pp 44–49
Cui K, Xing G, Liu X, Xing G, Wang Y (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci 146:9–16
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Davis BJ (1964) Disk electrophoresis II. Method and application to human serum proteins. Ann NY Acad Sci USA 121:404–427
De Souza IRP, MacAdam JW (1998) A transient increase n apoplastic peroxidase activity precedes decrease in elongation rate of B73 maize (Zea mays) leaf blades. Physiol Plant 104:556–562
Densay JPS, Sacciiar RC (1982) Hormonal control of peroxidase and its relationship with growth in mung bean seedlings. Plant Sci Lett 26:251–256
Dučić T, Lirić-Rajlić I, Mitrović A, Radotić K (2003/2004) Expression of antioxidant systems in Chenopodium rubrum seed germination. Biol Plant 47:527–533
Earnshaw BA, Johnson MA (1987) Control of wild carrot somatic embryo development by antioxidants. Plant Physiol 85:273–276
Edreva A (1988) New aspects of plant peroxidases-metabolic and physiological functions and applications as markers in biological and breeding research. Genetic and Breeding, No.5, 458–467 (In Bulg.). embryos. Plant Physiol Biochem 43:760–769
Frank T, Kevers C, Gaspar T (1995) Protective enzymatic systems against activated oxygen species compared in normal and hyperhydric shoots of Prunus avium L. raised in vitro. Plant Growth Regul 16:253–256
Frank T, Kevers C, Penel C, Greppin H, Housman H, Gaspar T (1998) Reducing properties, and markers of lipid peroxidation in normal and hyperhydrating shoots Prunus avium L. J Plant Physiol 153:339–346
Gaspar T (1995) The concept of cancer in in vitro plant cultures and the implication of habituation to hormones and hyperhydricity. Plant Tissue Cult Biotechnol 1:126–136
Gaspar T, Wyndacle R, Bouchet M, Ceulemans E (1977) Peroxidase and amylase activities in relation to germination of dormant and non-dormant wheat. Physiol Plant 40:11–14
Genkov T, Ivanova I (1995) Effect of cytokinin-active phenylurea derivatives on shoot multiplication, peroxidase and superoxide dismutase activities of in vitro cultured carnation. Bulg J Plant Physiol 21(1):73–83
Gupta SD, Datta S (2003/2004). Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol Plant 47:179–183
Halliwell B, Gutteridge JMC (1986) Iron and free radical reactions: two aspects of antioxidant protection. Trends Biochem Sci 11:375
Huan LVT, Takamura T, Tanaka M (2004) Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci 166:1443–1449
Hunt MD, Steffens JC (1994) Polyphenol oxidase (PPO) expression and insect resistance in transgenic tomato. J Cell Biochem Suppl 18A:88
Ichihashi S, Hiraiwa H (1996) Effect of solidifier, coconut water and carbohydrate source on growth of embryogenic callus in Phalaenopsis and allied genera. J Orchid Soc India 10:81–88
Ishii Y, Takamura T, Goi M, Tanaka M (1998) Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep 17:466–470
Jariteh M, Ebrahimzadeh H, Niknam V, Vahdati K, Amiri R (2011) Antioxidant enzymes activities during secondary somatic embryogenesis in Persian walnut (Juglans regia L.). Afr J Biotechnol 10(20):4093–4099
Joy RW, Patel KR, Thorpe TA (1988) Ascorbic acid enhancement of organogenesis in tobacco callus. Plant Cell Tissue Organ Cult 13:219–228
Junaid A, Mujib A, Sharma MP, Tang W (2007) Growth regulators affect primary and secondary somatic embryogenesis in Madagaskar periwinkle (Catharanthus roseus (L.) G. Don) at morphological and biochemical levels. Plant Growth Regul 51:271–281
Kairong C, Gengsheng X, Xinmin L, Gengmei X, Yafu W (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci 146:9–16
Kerbauy GB (1984) Plant regeneration of Oncidium varicosum (Orchidaceae) by means of root tip culture. Plant Cell Rep 3:27–29
Khan MH, Panda SK (2002) Induction of oxidative stress in roots of Oryza sativa L. in response to salt stress. Biol Plant 45:625–627
Krishnan PN, Latha PG, Seeni S (1993) Biochemical changes during protocorm formation from in vitro Growth embryos of Spathoglottis plicata Blume. J Orchid Soc India 7(1, 2):87–91
Krsnik-Rasol M (1991) Peroxidase as a developmental marker in plant tissue culture. Int J Dev Biol 35(3):259–263
Kumar GNM, Knowles NR (1993) Changes in lipid peroxidation and lipolytic and free radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol 102:115–124
Kumaria S, Tandon P (2000) Effect of growth regulators on peroxidase, polyphenol oxidase and IAA-oxidase activities and phenolic contents during protocorm development of Dendrobium fimbriatum var. oculatum Hk. f. J Orchid Soc India 14(1–2):27–39
Kumaria S, Tandon P (2004) Nucleic acids and soluble protein contents at different stages of protocorm development of Dendrobium fimbriatum var. oculatum Hk.f. as influenced by different growth regulators. Biosci Biotech Res Asia 02(1):65–70
Kumaria S, Chrungoo NK, Tandon P (1990) Activities of some oxidative enzymes in axenic cultures of protocorms of Cymbidium giganteum Wall as influenced by different growth regulators. J Orchid Soc India 4(1–2):37–44
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larson RA (1988) The antioxidants of higher plants. Phytochem 27:969–978
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Libik M, Konieczny R, Pater B, Slesak I, Miszalski Z (2005) Differences in the activities of some antioxidant enzymes and in H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep 23:834–841
Macko V, Staples RC, Yaniv Z, Granados RR (1976) Self-inhibitors of fungal spore germination. In: Weber DJ, Hess WM (eds) Form and function in the fungal spore. Wiley, New York, pp 73–100
Mahadevan A (1974) Methods in physiological plant pathology. Sivakami Publications, Madras
Manibhushanrao K, Zuber M, Matsuyama N (1988) Phenol metabolism and plant disease resistance. Acta Phytopathol Entomol Hung 23(1/2):103–114
Matters GL, Scandalios JG (1986) Effect of elevated temperature on catalase and superoxide dismutase during maize development. Differentiation 30:190–196
Mazzafera P, Robinson SP (2000) Characterization of polyphenol oxidase in coffee. Phytochem 55:256–296
Melo GA, Shimizu MM, Mazzafera P (2006) Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf miner and coffee leaf rust. Phytochem 67:277–285
Miller CO (1985) Possible regulatory roles of cytokinins NADH oxidation by peroxidase and a copper interaction. Plant Physiol 79:908–910
Mitrović A, Bogdanović J (2008) Activities of antioxidative enzymes during Chenopodium rubrum L. ontogenesis in vitro. Arch Biol Sci 60:223–231
Mittler R (2002) Oxidative stress; antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis AN, Dimassi K, Diamantidis G, Therios I (2004) Changes in peroxidases and catalase activity during in vitro rooting. Biol Plant 48:1–5
Paul S, Kumaria S, Tandon P (2012) An effective nutrient medium for asymbiotic seed germination and large scale in vitro regeneration of Dendrobium hookerianum a threatened orchid of northeast India. AOB Plants. doi:10.1093/aobpla/plr012.(Oxford)
Pitel JA, Cheliak WM, Wang BSP (1984) Changes in isozyme patterns during imbibitions and germination of lodgepole pine (Pinus contorta var. latifolia). Can J For Res 14:743–746
Ponting JD, Joslyn MA (1948) Ascorbic acid oxidation and browing in apple tissue extracts. Arch Biochem Biophys 19:47–63
Price AH, Atherton NM, Hendry GAF (1989) Plants under drought-stress generate activated oxygen. Free Radic Res Commun 8:61–66
Prodanović O, Prodanović R, Bogdanović J, Mitrović A, Milosavić N, Radotić K (2007) Antioxidative enzymes during germination of two lines of serbian spruce [Picea omorika (Panč.) Purkynĕ]. Arch Biol Sci 59:209–216
Quiles MJ, Lopez NI (2004) Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant growth. Effects on the chloroplast NADH dehydrogenase complex. Plant Sci 166:815–823
Rajeswari V, Paliwal K (2008) Peroxidase and catalase changes during in vitro adventitious shoot organogenesis from hypocotyls of Albizia odoratissima L.f. (Benth). Acta physiol Plant 30:825–832
Richardson KA, Peterson RL, Currah RS (1992) Seed reserves and early symbiotic protocorm development of Planthera hyperborean (Orchidaceae). Can J Bot 70:291–300
Roberts EH (1972) Oxidative processes and the control of seed germination. In: Heydecker W (ed) Seed ecology. Butterworts, London, pp 189–218
Roupakias DC, McMillin DE, Scandalios JC (1980) Chromosomal location of the catalase structural genes in Zea mays using B-A translocations. Theor Appl Genet 58:211–218
Scandalios JG (1974) Isozymes in development and differentiations. Ann Rev Plant Physiol 25:225–258
Scandalios JG (1987) The antioxidant enzyme genes cat and sod of maize: regulation, functional significance, and molecular biology. In: Rattazzi MC, Scandalios JG, Whitt GS (eds) Isozymes. Current topics in biological and medical research, molecular and cellular biology, vol 14. Alan R. Liss, New York, pp 19–44
Scandalios JG (1990) Response of plant antioxidant defense genes. In: Scandalios JG, Wright T (eds) Genomic responses to environmental stress. Academic, New York, pp 1–41
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellins, and ascorbic acid. Plant Physiol 125:1591–1602
Sharma J (1998) Studies on Vanda: effect of age of capsules on in vitro seed germination. J Orchid Soc India 12(12):43–45
Shi C, Dai Y, Xu X, Xie Y, Liu Q (2002) The purification of polyphenol oxidase from tobacco. Protein Expr Purif 24:51–55
Steward FC, Mapes MO (1971) Morphogenesis in aseptic cell culture of Cymbidium. Bot Gaz 132:65–70
Sung ZR, Okimoto R (1983) Coordinate gene expression during somatic embryogenesis in Carrot. Proc Natl Acad Sci 80:2661–2665
Tang W, Newton RJ (2005) Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobes L.) zygotic embryos. Plant Physiol Biochem 43:760–769
Teixeira da Silva JA, Singh N, Tanaka M (2006) Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tiss Organ Cult 84:135–144
Thakar J, Bhargava S (1999) Seasonal variation in antioxidant enzymes and the sprouting response of Gmelina arborea Roxb. Nodal sectors cultured in vitro. Plant Cell Organ Cult 59:181–187
Tian M, Gu Q, Zhu M (2003) The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci 165:701–707
Van Breusegem F, Vranova E, Dat JF, Inze D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Van Loon LC, Geelen JLMC (1971) The relation of polyphenol oxidase and peroxidase to symptom expression in tobacco var. “Samsun NN” after infection with tobacco mosaic virus. Acta phytopathol Acad Sci Hung 6:9–20
Vatankhah E, Niknam V, Ebrahimzadeh M (2010) Activity of antioxidant enzyme during in vitro organogenesis in Crocus Sativus. Biol Plant 54(3):509–514
Woodbury W, Spencer AK, Stahman MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electronic J Biotech 13:1–9
Zhao P, Wu F, Feng FS, Wang WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from callus culture of Dendrobium candidum Wall Ex Lindl. In Vitro Cell Dev Biol Plant 44:178–185
Acknowledgments
Sumi Paul acknowledges the financial support under the meritorious fellowship from the University Grant Commission, India. She would also like to acknowledge Miss Purnima Devi for helping in arranging the photographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Prasad.
Rights and permissions
About this article
Cite this article
Paul, S., Kumaria, S. & Tandon, P. Comparative study on the changes of proteins and oxidative enzymes occurring in protocorms and protocorm-like bodies systems of development in the orchid Dendrobium hookerianum . Acta Physiol Plant 36, 2113–2123 (2014). https://doi.org/10.1007/s11738-014-1588-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1588-7