Abstract
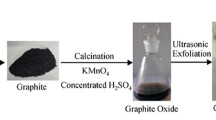
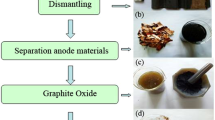
Soluble graphene nanosheets are fabricated from recycled graphite of spent lithium ion batteries through a modified Hammers process followed by deoxygenation with NaOH–KOH eutectic. Ultrasonic exfoliation in N-methyl-pyrrolidone indicates the loosened graphene layers in recycled graphite are prone to exfoliation. Reduction of the exfoliated graphene oxide sheets was conducted in molten NaOH–KOH eutectic at different temperatures. The results show that molten NaOH–KOH effectively eliminates the unsaturated oxygen-containing moieties from the exfoliated graphene oxide sheets while creating more hydroxyl functional groups. Higher temperature treatment is more prone to remove hydroxyls while producing the shrinkage on the surface of graphene sheets. Graphene sheet with a good solubility is produced when the graphene oxide is heat-treated at 220 °C for 10 h. After reduction, the graphene oxide sheets exhibit excellent dispersibility or solubility in water, ethanol and other polar solvents, therefore being highly desirable for solution processing of graphene materials. Such study not only identifies a high-quality stockpile to prepare soluble graphene but also paves a feasible alternative of graphite recycling from spent lithium batteries.







Similar content being viewed by others
References
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, and A.A. Firsov, Electric Field Effect in Atomically Thin Carbon Films, Science, 2004, 306(6996), p 666–669
C.N.R. Rao, A.K. Sood, K.S. Subrahmanyam, and A. Govindaraj, Graphene: The New Two-Dimensional Nanomaterial, Angew. Chem. Int. Ed., 2009, 48(42), p 7752–7777
M. Balasubramaniam and S. Balakumar, Tri-Solvent Mediated Probing Of Ultrasonic Energy Towards Exfoliation of Graphene Nanosheets for Supercapacitor Application, Mater. Lett., 2016, 182, p 63–67
C.M. Seah, B. Vigolo, S.P. Chai, and A.R. Mohamed, Mechanisms of Graphene Fabrication Through Plasma-Induced Layer-by-Layer Thinning, Carbon, 2016, 105, p 496–509
T. Qian, C.F. Yu, S.S. Wu, and J. Shen, In situ Polymerization of Highly Dispersed Polypyrrole on Reduced Graphite Oxide for Dopamine Detection, Biosens. Bioelectron., 2013, 50, p 157–160
L. Liu, Z.G. Shen, S.S. Liang, M. Yi, X.J. Zhang, and S.L. Ma, Graphene for Reducing Bubble Defects and Enhancing Mechanical Properties of Graphene/Cellulose Acetate Composite Films, J. Mater. Sci., 2014, 49, p 321–328
P. Ganguly, R.K. Kotnala, S. Singh, R.P. Pant, and N. Singh, Graphene Functionalized with 3-Mercatopropionic Acid Capped Zinc Peroxide Nanoparticles: A Potential Ferromagnetic Material at Room-Temperature, Carbon, 2015, 95, p 428–433
K. Krishnamoorthy, G.S. Kim, and S.J. Kim, Graphene Nanosheets: Ultrasound Assisted Synthesis and Characterization, Ultrason. Sonochem., 2013, 20(1), p 644–649
T.D. Dao, H.I. Lee, H.M. Jeong, and B.K. Kim, Direct Covalent Modification of Thermally Exfoliated Graphene Forming Functionalized Graphene Stably Dispersible in Water and poly(Vinyl Alcohol), Colloid Polym. Sci., 2013, 291(10), p 2365–2374
S.M. Notley, Highly Concentrated Aqueous Suspensions of Graphene Through Ultrasonic Exfoliation with Continuous Surfactant Addition, Langmuir, 2012, 28(40), p 14110–14113
S. Yoon and I. In, Role of poly(N-vinyl-2-pyrrolidone) as Stabilizer for Dispersion of Graphene via Hydrophobic Interaction, J. Mater. Sci, 2011, 4695, p 1316–1321
X.Q. Liang, J. Guo, S.Y. Liang, C.H. Hong, and Y.D. Zhao, Synthesis of Soluble Graphene Nanosheets from Graphite Fluoride in Low-Temperature Molten Hydroxides, Mater. Lett., 2014, 135, p 92–95
X.J. Zhou, J.L. Zhang, H.X. Wu, H.J. Yang, J.Y. Zhang, and S.W. Guo, Reducing Graphene Oxide via Hydroxylamine: a Simple and Efficient Route to Graphene, J. Phys. Chem. C, 2011, 115(24), p 11957–11961
S.P. Lonkar, Y.S. Deshmukh, and A.A. Abdala, Recent Advances in Chemical Modifications of Graphene, Nano Res., 2015, 8(4), p 1039–1074
D. Li, M.B. Muller, S. Gilje, R.B. Kaner, and G.G. Wallace, Processable Aqueous Dispersions of Graphene Nanosheets, Nat. Nanotechnol., 2008, 3(2), p 101–105
X.B. Fan, W.C. Peng, Y. Li, X.Y. Li, S.L. Wang, G.L. Zhang, and G.B. Zhang, Deoxygenation of Exfoliated Graphite Oxide Under Alkaline Conditions: A Green Route to Graphene Preparation, Adv. Mater., 2008, 20(23), p 4490–4493
Y. Matsuo, K. Fumita, T. Fukutsuka, Y. Sugie, H. Koyama, and K. Inoue, Butyrolactone Derivatives as Electrolyte Additives For Li-Ion Batteries with Graphite Anode, J. Power Sources, 2003, 119–121(2), p 373–377
M.J. McAllister, J.L. Li, D.H. Adamson, H.C. Schniepp, A.A. Abdala, J. Liu, M. Herrera-Alonso, D.L. Milius, R. Car, R.K. Prudhomme, and I.A. Aksay, Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite, Chem. Mater., 2007, 19(18), p 4396–4404
A.B. Bourlinos, V. Georgakilas, R. Zboril, D. Jancik, M.A. Karakassides, A. Stassinopoulos, D. Anglos, and E.P. Giannelis, Reaction of Graphite Fluoride with NaOH–KOH Eutectic, J. Fluor. Chem., 2008, 129(8), p 720–724
W.K. Li and Y.J. Yang, The Reduction of Graphene Oxide by Elemental Copper and its Application in the Fabrication of Graphene Supercapacitor, J. Solid State Electrochem., 2014, 18(6), p 1621–1626
W. Gao, L.B. Alemany, L.J. Ci, and P.M. Ajayan, New Insights into the Structure and Reduction of Graphite Oxide, Nat. Chem., 2009, 1(5), p 403–408
Y. Wang, D.C. Alsmeyer, and R.L. McCreery, Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra, Chem. Mater., 1990, 2(5), p 557–563
J. Zheng, H.T. Liu, B. Wu, Y.L. Guo, T. Wu, G. Yu, Y.Q. Liu, and D.B. Zhu, Production of Graphene Nanospheres by Annealing of Graphene Oxide in Solution, Nano. Res., 2011, 4(7), p 705–711
Y.X. Xu, H. Bai, G.W. Lu, C. Li, and G.D. Shi, Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets, J. Am. Chem. Soc., 2008, 130(18), p 5856–5857
L.L. Dong, W.G. Chen, N. Deng, and C.H. Zheng, A Novel Fabrication of Graphene by Chemical Reaction with Green Reductant, Chem. Eng. J., 2016, 306, p 754–762
S. Dubin, S. Gilje, K. Wang, V.C. Tung, K. Cha, A.S. Hall, J. Farrar, R. Varshneya, Y. Yang, and R.B. Kaner, A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents, ACS Nano, 2010, 4(7), p 3845–3852
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, L., Liu, X., Wan, C. et al. Soluble Graphene Nanosheets from Recycled Graphite of Spent Lithium Ion Batteries. J. of Materi Eng and Perform 27, 875–880 (2018). https://doi.org/10.1007/s11665-018-3156-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3156-6