Abstract
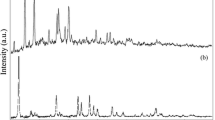
A facile route to synthesize copper indium diselenide (CuInSe2) nanoparticles in aqueous medium was developed using mercaptoacetic acid (MAA) as capping agent. Two different mole ratios (5 and 10) of MAA were used to synthesize CuInSe2 nanoparticles at room temperature, as well as hydrothermal (high temperature) method. Powder x-ray diffraction analysis reveals that the nanoparticles exhibit chalcopyrite phase and the crystallinity increases with increasing the capping ratio. Raman analysis shows a strong band at 233 cm−1 due to the combination of B2 (E) modes. Broad absorption spectra were observed for the synthesized CuInSe2 nanoparticles. The effective surface capping by MAA on the nanoparticles surface was confirmed through attenuated total reflection–Fourier transform infrared spectral analysis. The thermal stability of the synthesized samples was analyzed through thermogravimetric analysis–differential scanning calorimetry. The change in morphology of the synthesized samples was analyzed through scanning electron microscope and it shows that the samples prepared at room temperature are spherical in shape, whereas hydrothermally synthesized samples were found to have nanorod- and nanoflake-like structures. Transmission electron microscope analysis further indicates larger grains for the hydrothermally prepared samples with 10 mol ratio of MAA. Comparative analyses were made for synthesizing CuInSe2 nanoparticles by two different methods to explore the role of ligand and influence of temperature.
Similar content being viewed by others
References
V. Lesnyak, N. Gaponik, and A. Eychmuller, Chem. Soc. Rev. 42, 2905 (2013).
A.M. Smith and S. Nie, Acc. Chem. Res. 43, 190 (2010).
L. Etger, Materials 6, 445 (2013).
J.H. Bang and P.V. Kamat, ACS Nano 3, 1467 (2009).
N. Al-Hosiny, S. Abdallah, A. Badawi, K. Easawi, and H. Talaat, Mater. Sci. Semicond. Process. 26, 238 (2014).
C. Pan, S. Niu, Y. Ding, L. Dong, R. Yu, Y. Liu, G. Zhu, and Z.L. Wang, Nano Lett. 12, 3302 (2012).
J.Y. Chang, L.F. Su, C.C. Chang, and J.M. Lin, Chem. Commun. 48, 4848 (2012).
W. Li, Z. Pan, and X. Zhong, J. Mater. Chem. A 3, 1649 (2015).
Y. Zhao and C. Burda, Energy Environ. Sci. 5, 5564 (2012).
X.Q. Chen, Z. Li, Y. Bai, Q. Sun, L.Z. Wang, and S.X. Dou, Chem. Eur. J. 21, 1055 (2015).
P.M. Allen and M.G. Bawendi, J. Am. Chem. Soc. 130, 9240 (2008).
W.N. Shafarman, R.W. Birkmire, D.A. Fardig, B.E. McCandless, A. Mondal, J.E. Phillips, and R.D. Varrin Jr, Sol. Cells 30, 61 (1991).
L. Stolt, J. Hedstrom, J. Kessler, M. Ruckh, K.O. Velthaus, and H.W. Schock, Appl. Phys. Lett. 62, 597 (1993).
J.L. Shay, S. Wagner, and H.M. Kasper, Appl. Phys. Lett. 27, 89 (1975).
L.L. Kazmerski and G.A. Sanborn, J. Appl. Phys. 48, 3178 (1977).
P. Jackson, D. Hariskos, E. Lotter, S. Paetel, R. Wuerz, R. Menner, W. Wischmann, and M. Powalla, Prog. Photovolt. Res. Appl. 19, 894 (2011).
S.J. Ahn, C.W. Kim, J.H. Yun, J. Gwak, S. Jeong, B.H. Ryu, and K. Yoon, J. Phys. Chem. C 114, 8108 (2010).
J. Xu, C.Y. Luan, Y.B. Tang, X. Chen, J.A. Zapien, W.J. Zhang, H.L. Kwong, X.M. Meng, S.T. Lee, and C.S. Lee, ACS Nano 4, 6064 (2010).
S.E. Wark, C.H. Hsia, Z. Luo, and D.H. Son, J. Mater. Chem. 21, 11618 (2011).
Y. Luo, Colloid J. 71, 375 (2009).
H. Chen, S.M. Yu, D.W. Shin, and J.B. Yoo, Nanoscale Res. Lett. 5, 217 (2010).
S.L. Castro, S.G. Bailey, R.P. Raffaelle, K.K. Banger, and A.F. Hepp, Chem. Mater. 15, 3142 (2003).
H. Peng, D.T. Schoen, S. Meister, X.F. Zhang, and Y. Cui, J. Am. Chem. Soc. 129, 34 (2007).
J.P. Park, S.K. Kim, J.Y. Park, K.M. Ok, and I.I.-W. Shim, Bull. Korean Chem. Soc. 30, 853 (2009).
H. Liu, Z. Jin, W. Wang, and J. Li, Cryst Eng Comm 13, 7198 (2011).
Y. Min, G.D. Moon, J. Park, M. Park, and U. Jeong, Nanotechnology 22, 465604 (2011).
B. Koo, R.N. Patel, and B.A. Korgel, J. Am. Chem. Soc. 131, 3134 (2009).
M. Singh, J. Jiu, T. Sugahara, and K. Suganuma, Thin Solid Films 565, 11 (2014).
J. Chang and E.R. Waclawik, RSC Adv. 4, 23505 (2014).
D. Aldakov, A. Lefrançois, and P. Reiss, J. Mater. Chem. C 1, 3756 (2013).
D. Wang, W. Zheng, C. Hao, Q. Peng, and Y. Li, Chem. Commun., 22, 2556 (2008).
J. Embden, A.S.R. Chesman, and J.J. Jasieniak, Chem. Mater. 27, 2246 (2015).
G.W. Kim, F.P.G. Arquer, Y.J. Yoon, X. Lan, M. Liu, O. Voznyy, Z. Yang, F. Fan, P. Kanjanaboos, S. Hoogland, J.Y. Kim, and E.H. Sargent, Nano Lett. 15, 7691 (2015).
J.V. Williams, C.N. Adams, N.A. Kotov, and P.E. Savage, Ind. Eng. Chem. Res. 46, 4358 (2007).
H. Zang, L. Wang, H. Xiong, L. Hu, B. Yang, and W. Li, Adv. Mater. 15, 1712 (2003).
J. Xing, T.P. Vinod, and J. Kim, J. Nanosci. Nanotechnol. 12, 5892 (2012).
S. Liu, H. Zhang, Y. Qiao, and X. Su, RSC Adv. 2, 819 (2012).
X. Hu, Q. Zhang, X. Huang, D. Li, Y. Luo, and Q. Meng, J. Mater. Chem. 21, 15903 (2011).
J. Zhu, A. Li, and Q. Yan, J. Nanosci. Nanotechnol. 10, 7519 (2010).
J. Ramkumar, S. Ananthakumar, and S. Moorthy Babu, Sol. Energy 106, 177–183 (2014).
P.W. Yu, J. Appl. Phys. 47, 677 (1976).
N. Katsuhiro, T. Omata, and S. Otsuka-Yao-Matsuo, J. Phys. Chem. C 113, 3455 (2009).
J. Alvarez-Garcia, B. Barcones, A. Perez-Rodriguez, A. Romano-Rodriguez, J.R. Morante, A. Janotti, S.H. Wei, and R. Scheer, Phys. Rev. B 71, 054303 (2005).
M. Ahmadi, S.S. Pramana, L. Xi, C. Boothroyd, Y.M. Lam, and S. Mhaisalkar, J. Phys. Chem. C 116, 8202 (2012).
Acknowledgements
The authors sincerely thank the Department of Science and Technology (DST), Govt. of India, for providing financial support under Solar Energy Research Initiative program (Project number: DST/TMC/SERI/FR/90). J. Ram Kumar and S. Ananthakumar sincerely thank the Ministry of New and Renewable Energy (MNRE), Govt. of India, for providing fellowships under the National Renewable Energy Fellowship (NREF) scheme␣for the doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram Kumar, J., Ananthakumar, S. & Moorthy Babu, S. Influence of Capping Ligand and Synthesis Method on Structure and Morphology of Aqueous Phase Synthesized CuInSe2 Nanoparticles. J. Electron. Mater. 46, 296–305 (2017). https://doi.org/10.1007/s11664-016-4906-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-016-4906-6