Abstract
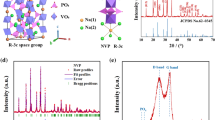
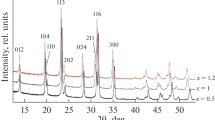
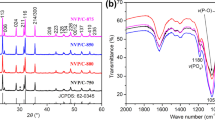
Rhombohedral Na3V2(PO4)3 with a Na+ superionic conductor structure was synthesized using a solid-state reaction method. Citric acid was used as a carbon resource for carbon-thermal reduction reaction to reduce the oxidation state of vanadium. The shape of Na3V2(PO4)3 particles is irregular and its average diameter is in the range 30–50 nm. The Na3V2(PO4)3 exhibits a superior cycling ability and rate capability. The discharge capacity retains 74.3% of the discharge capacity of its first cycle with coulombic efficiency of 99.3% after 100 cycles. The discharge capacity of Na3V2(PO4)3 at 10 C is 48.87 mAh g−1, which is 58.4% of the cell cycled at 0.1 C. Furthermore, the structure of Na3V2(PO4)3 is stable for a considerable amount of Na+ ions (2 mol of Na+ ions) insertion and extraction with only 0.42% difference of unit-cell volume between fully charged and discharged states. Na3V2(PO4)3 is a potential cathode material for sodium-ion battery applications.
Similar content being viewed by others
References
D. Hamani, M. Ati, J.M. Tarascon, and P. Rozier, Electrochem. Commun. 13, 938 (2011).
J.J. Ding, Y.N. Zhou, Q. Sun, and Z.W. Fu, Electrochem. Commun. 22, 85 (2012).
J.J. Ding, Y.N. Zhou, Q. Sun, X.Q. Yu, X.Q. Yang, and Z.W. Fu, Electrochim. Acta 87, 388 (2013).
J. Molenda and A. Stoklosa, Solid State Ionics 38, 1 (1990).
W. Song and S. Liu, Solid State Sci. 15, 1 (2013).
R. Gover, A. Bryan, P. Burns, and J. Barker, Solid State Ionics 177, 1495 (2006).
W. Song, X. Ji, Z. Wu, Y. Yang, Z. Zhou, F. Li, Q. Chen, and C.E. Banks, J. Power Sources 256, 258 (2014).
K. Saravanan, C.W. Mason, A. Rudola, K.H. Wong, and P. Balaya, Adv. Energy Mater. 3, 444 (2013).
S.M. Oh, S.T. Myung, J. Hassoun, B. Scrosati, and Y.K. Sun, Electrochem. Commun. 22, 149 (2012).
A. Sun, F.R. Beck, D. Haynes, J.A. Poston, S.R. Narayanan, P.N. Kumta, and A. Manivannan, Mater. Sci. Eng. B 177, 1729 (2012).
J. Barker, M.Y. Saidi, and J.L. Swoyer, Electrochem. Solid-State Lett. 6, A1 (2003).
H. Zhuo, X. Wang, A. Tang, Z. Liu, S. Gamboa, and P.J. Sebastian, J. Power Sources 160, 698 (2006).
Z. Liu, X. Wang, Y. Wang, A. Tang, and S. Yang, Trans. Nonferrous Met. Soc. China 18, 346 (2008).
T. Ramireddy, M.M. Rahman, N. Sharma, A.M. Glushenkov, and Y. Chen, J. Power Sources 271, 497 (2014).
S. Zhang, C. Deng, and Y. Meng, J. Mater. Chem. A 2, 20538 (2014).
Z. Jian, L. Zhao, H. Pan, Y.S. Hu, H. Li, W. Chen, and L. Chen, Electrochem. Commun. 14, 86 (2012).
S.J. Lim, D.W. Han, D.H. Nam, K.S. Hong, J.Y. Eom, W.H. Ryu, and H.S. Kwon, J. Mater. Chem. A 2, 19623 (2014).
Z. Jian, H. Yu, and H. Zhou, Electrochem. Commun. 34, 215 (2013).
L. Si, Z. Yuan, L. Hu, Y. Zhu, and Y. Qian, J. Power Sources 272, 880 (2014).
G. Li, D. Jiang, H. Wang, X. Lan, H. Zhong, and Y. Jiang, J. Power Sources 265, 325 (2014).
H. Li, Y. Bai, F. Wu, Y. Li, and C. Wu, J. Power Sources 273, 784 (2015).
K. Du, H. Guo, G. Hu, Z. Peng, and Y. Cao, J. Power Sources 223, 284 (2013).
T. Jiang, W. Pan, J. Wang, X. Bie, F. Du, Y. Wei, C. Wang, and G. Chen, Electrochim. Acta 55, 3864 (2010).
P. Barpanda, T. Ye, S. Nishimura, S.C. Chung, Y. Yamada, M. Okubo, H. Zhou, and A. Yamada, Electrochem. Commun. 24, 116 (2012).
X. Ma, H. Chen, and G. Ceder, J. Electrochem. Soc. 158, A1307 (2011).
P. Vassilaras, X. Ma, X. Li, and G. Ceder, J. Electrochem. Soc. 160, A207 (2012).
Y.Q. Qiao, X.L. Wang, J.Y. Xiang, D. Zhang, W.L. Liu, and J.P. Tu, Electrochim. Acta 56, 2269 (2011).
I.V. Zatovsky, Acta Crystallogr. Sect. E Struct. Rep. Online 66, i12 (2010).
A. Caballero, L. Hernán, J. Morales, L. Sánchez, J.S. Peña, and M.A.G. Aranda, J. Mater. Chem. 12, 1142 (2002).
B. Mortemard de Boisse, D. Carlier, M. Guignard, and C. Delmas, J. Electrochem. Soc. 160, A569 (2013).
M. Sathiya, K. Hemalatha, K. Ramesha, J.M. Tarascon, and A.S. Prakash, Chem. Mater. 24, 1846 (2012).
N. Van Nghia, P.W. Ou, and I.M. Hung, Electrochim. Acta 161, 63 (2015).
Acknowledgements
The authors acknowledge the financial support from the National Science Council in Taiwan under Contract Nos. NSC 103-2623-E-155-002-ET and MOST 104-2623-E-155-005-ET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Nghia, N., Jafian, S. & Hung, IM. Synthesis and Electrochemical Performance of the Na3V2(PO4)3 Cathode for Sodium-Ion Batteries. J. Electron. Mater. 45, 2582–2590 (2016). https://doi.org/10.1007/s11664-016-4425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-016-4425-5