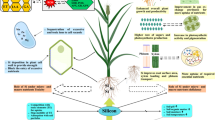
Abstract
An effective protocol for adventitious shoot regeneration with reduced hyperhydricity was established from proximal cotyledon explants of Citrullus lanatus (Thunb.) cv. Arka Manik. The highest frequencies of shoot regeneration (100%) and shoots per explant (13.13 ± 0.29) occurred in Murashige and Skoog (MS) medium supplemented with 8.88 μM 6-benzylaminopurine and 2.85 μM indole-3-acetic acid. The proportions of adventitious shoots that were hyperhydric ranged from 8.3 to 35.3%. To reduce the occurrence of hyperhydric shoots, shoot regeneration medium was supplemented with silver ions in the form of silver nitrate or silver thiosulfate. Amendment of the regeneration medium with silver ions was found to be essential during the shoot initiation and elongation phases for the development of normal shoots. Supplementation with 9.0 μM silver nitrate resulted in the greatest reduction of hyperhydricity (to 5.8%) and increased number of shoots (to 14.20 ± 0.36). The highest rate of rooting (80%) was observed on MS medium supplemented with 4.92 μM indole-3-butyric acid, and rooted shoots from this treatment showed 3.97 ± 0.16 roots/shoot. Over 85% of the regenerated plants survived acclimatization in red soil:sand (1:1 v/v) and transferred to soil mixtures viz, garden soil, farmyard soil, and sand (1:1:1, v/v/v). This protocol describes the positive influence of silver ions in reducing the hyperhydricity of watermelon shoot cultures and thus could be used for genetic transformation protocols.



Similar content being viewed by others
References
Adelberg J, Rhodes BB, Skorupska H (1990) Generating tetraploid melons from tissue culture. HortSci 25:73
Akashi K, Morikawa K, Yokota A (2005) Agrobacterium-mediated transformation system for the drought and excess light stress-tolerant wild watermelon (Citrullus lanatus). Plant Biotechnol 22:13–18
Ananthakrishnan G, Xia X, Elman C, Singer S, Paris HS, Galon A, Gaba V (2003) Shoot production in squash (Cucurbita pepo) by in vitro organogenesis. Plant Cell Rep 21:739–746
Anonymous (1992) In: The wealth of India-A dictionary of Indian raw materials and industrial products, Vol. 3. Ca-Ci. New Delhi: Publication and Information Directorate, CSIR; pp:606–609
Badr-Elden AM, Nower AA, Ibrahim IA, Ebrahim MHK, Elazeim TMA (2012) Minimizing the hyperhydricity associated with in vitro growth and development of watermelon by modifying culture conditions. Afr J Biotechnol 11:8705–8717
Bais HP, Sudha G, Ravishankar GA (2000) Putrescine and silver nitrate influences shoot multiplication, in vitro flowering and endogenous titres of polyamines in Cichorium intybus L. cv. Lucknow local. J Plant Growth Regul 19:238–248
Bais HP, Sudha G, Ravishankar GA (2001) Influence of putrescine, silver nitrate and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Cichorium intybus and their regenerants. Plant Cell Rep 20:547–555
Baskaran P, Moyo M, Van Staden J (2014) In vitro plant regeneration, phenolic compound production and pharmacological activities of Coleonema pulchellum. South Afr J Bot 90:74–79
Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Chakrabarty D, Park SY, Ali MB, Shin KS, Paek KY (2006) Hyperhydricity in apple: ultrastructural and physiological aspects. Tree Physiol 26:377–388
Chaturvedi R, Bhatnagar SP (2001) High-frequency shoot regeneration from cotyledon explants of watermelon cv. sugar baby. In Vitro Cell Dev Biol - Plant 37:255–258
Choi PS, Soh WY, Kim YS, Yoo OJ, Liu JR (1994) Genetic transformation and plant regeneration of watermelon using Agrobacterium tumefaciens. Plant Cell Rep 13:344–348
Compton ME (2000) Interaction between explant size and cultivar impacts shoot organogenic competence of watermelon cotyledon. HortSci 35:749–750
Compton ME, Gray DJ (1991) Shoot organogenesis on cotyledons of watermelon. HortSci 26:772
Compton ME, Gray DJ (1993) Shoot organogenesis and plant regeneration from cotyledons of diploid, triploid and tetraploid watermelon. J Am Soc Hortic Sci 118:151–157
Compton ME, Gray DJ (1994) Adventitious shoot organogenesis and plant regeneration from cotyledons of tetraploid watermelon. HortSci 29:211–213
Compton ME, Gray DJ (1999) Shoot organogenesis from cotyledon explants of watermelon. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercises, 2nd edn. CRC Press, Boca Raton, pp 149–157
Compton ME, Gray DJ, Gaba VP (2004) Use of tissue culture and biotechnology for the genetic improvement of watermelon. Plant Cell Tiss Org Cult 77:231–243
Curuk S, Elman C, Schlarman E, Sagee O, Shomer I, Cetiner S, Gray DJ, Gaba V (2002) A novel pathway for rapid shoot regeneration from the proximal zone of the hypocotyls of melon (Cucumis melo L.). In Vitro Cell Dev Biol - Plant 38:260–267
Dong JZ, Jia SR (1991) High efficiency plant regeneration from cotyledons of watermelon (Citrullus vulgaris Schrad.). Plant Cell Rep 9:559–562
Eapen S, George L (1997) Plant regeneration from peduncle segments of oil seed Brassica species: influence of silver nitrate and silver thiosulfate. Plant Cell Tiss Org Cult 51:229–232
Ellul P, Rios G, Atare A, Roig LA, Serrano R, Moreno V (2003) The expression of Saccharomyces cerevisiae HAL1 gene increases salt tolerance in transgenic watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai.]. Theor Appl Genet 107:462–469
Evans PT, Malmburg RL (1989) Do polyamines have a role in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
Ganasan K, Huyop F (2010) In vitro regeneration of Citrullus lanatus cv. Round Dragon J Biol Sci 10:131–137
Gaspar T, Kevers C, Franck T, Bisbis B, Billard JP, Huault C, Le Dily F, Penel C, Crevecoeur M, Greppin H (1995) Paradoxical results in the analysis of hyperhydric tissues considered as being under stress: questions for a debate. Bulg J Plant Physiol 21:80–97
George EF (1996) Plant propagation by tissue culture, part 2: in practice. Exegetics LTD, Edington
Hall CV (2004) Watermelons as food in the 22 century. In: Nath P, Gaddagimath PB, Dutta OP (eds) Food security and vegetables: a global perspective. Dr. Prem Nath Agricultural Science Foundation, India, pp 135–148
Hao LX, Wang HM (1998) A study on building up the regeneration system of watermelon. Acta Agric Bor Sin 13:112–115
Huang YC, Chiang CH, Li CM, Yu TA (2011) Transgenic watermelon lines expressing the nucleocapsid gene of Watermelon silver mottle virus and the role of thiamine in reducing hyperhydricity in regenerated shoots. Plant Cell Tiss Org Cult 106:21–29
Hyde CL, Phillips GC (1996) Silver nitrate promotes shoot development and plant regeneration of chili pepper (Capsicum annuum L.) via organogenesis. In Vitro Cell Dev Biol - Plant 32:72–80
Ivanova M, van Staden J (2008) Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tiss Org Cult 92:227–231
Ivanova M, Novak O, Strnad M, van Staden J (2006) Endogenous cytokinins in shoots of Aloe polyphylla cultured in vitro in relation to hyperhydricity, exogenous cytokinins and gelling agents. Plant Growth Regul 50:219–230
Kataeva NV, Alexanandrova IG, Butenko RG, Dragavtceva EV (1991) Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tiss Org Cult 14:31–40
Kevers C, Franck T, Strasser RJ, Dommes J, Gaspar T (2004) Hyperhydricity of micropropagated shoots: a typically stress-induced change of physiological state. Plant Cell Tiss Org Cult 77:181–191
Kim JY, Yi YK, Song YH (1998) Plant diseases on green-house crops in Kyeongbuk areas. Kor J Plant Pathol 14:41–45
Lentini Z, Mussell H, Mutschler MA, Earle ED (1988) Ethylene generation and reversal of ethylene effects during development in vitro rapid-cycling Brassica campestris L. Plant Sci 54:75–81
Li J, Li XM, Qin YG, Tang Y, Wang L, Ma C, Li HX (2011) Optimized system for plant regeneration of watermelon (Citrullus lanatus Thunb). Afr J Biotechnol 10:9760–9765
Lin CY, Ku HM, Chiang YH, Ho HY, Yu TA, Jan FJ (2012) Development of transgenic watermelon resistant to Cucumber mosaic virus and Watermelon mosaic virus by using a single chimeric transgene construct. Transgenic Res 21:983–993
Majada JP, Fal M, Sanchez-Tames R (1997) The effect of ventilation rate on proliferation and hyperhydricity of Dianthus caryophyllus L. In Vitro Cell Dev Biol - Plant 33:62–69
Mayor ML, Nestares G, Zorzoli R, Picardi LA (2003) Reduction of hyperhydricity in sunflower tissue culture. Plant Cell Tiss Org Cult 72:99–103
Miyazaki JH, Yang SF (1987) The methionine salvage pathway in relation to ethylene and polyamine biosynthesis. Physiol Plantarum 69:366–370
Mohiuddin AKM, Chowdhury MKU, Abdullah ZC, Napis S (1997) Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tiss Org Cult 51:75–78
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Park SM, Lee JS, Jenal S, Jeon SL, Shin YS, Her NH, Lee JH, Lee M, Ryu KH, Yang SG, Harn CH (2005) Transgenic watermelon rootstock resistant to CGMMV (Cucumber green mottle mosaic virus) infection. Plant Cell Rep 24:350–356
Pirinc V, Onay A, Yildirim H, Adiyaman F, Isikalan C, Basaran D (2003) Adventitious shoot organogenesis and plant regeneration from cotyledons of diploid Diyarbakir watermelon (Citrullus lanatus cv. Surme). Turk J Biol 27:101–105
Qin Y, Zhang S, Zhang L, Zhu D, Syed A (2005) Response of in vitro strawberry to silver nitrate (AgNO3). HortSci 40:747–751
Roustan JP, Latche A, Fallot J (1990) Inhibition of ethylene production and simulation of carrot somatic embryogenesis by salicylic acid. Biol Plantarum 32:273–276
Sen A, Alikamanoglu S (2013) Antioxidant enzyme activities, malondialdehyde, and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture. In Vitro Cell Dev Biol - Plant 49:396–404
Songstad DD, Duncan DR, Widholm JM (1988) Effect of 1-aminocyclopropane-1-carboxylic acid, silver nitrate and norbornadiene on plant regeneration from maize callus cultures. Plant Cell Rep 7:262–265
Srivastava DR, Andrianov VM, Piruzian ES (1989) Tissue culture and plant regeneration of watermelon (Citrullus vulgaris Schrad. cv. Melitopolski). Plant Cell Rep 8:300–302
Sultana RS, Bari MA (2003) Effect of different plant growth regulators on direct regeneration of watermelon. Plant Tiss Cult 13:173–177
Tabart J, Franck T, Kevers C, Dommes J (2015) Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots. Plant Cell Tiss Org Cult 120:11–18
Tabei Y, Yamanaka H, Tsuguo K (1993) Adventitious shoot induction and plant regeneration from cotyledons of mature seed in watermelon (Citrullus lanatus L.). Plant Tiss Cult Lett 10:235–241
Wang HZ, Zhao PJ, Xu JC, Zhao H, Zhang HS (2003) Virus resistance in transgenic watermelon plants containing a WMV-2 coat protein gene. J Genet Genomics 30:70–75
Williams RR, Taji AM (1991) Effect of temperature, gel concentration and cytokinins on vitrification of Olearia microdisca (J.M. Black) in vitro shoot cultures. Plant Cell Tiss Org Cult 26:1–6
Yang SF (1985) Biosynthesis and action of ethylene. HortSci 20:41–45
Zhao XC, Qu X, Mathews DE, Schaller GE (2002) Effect of ethylene-pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol 130:1983–1991
Zobayed SMA, Armstrong J, Armstrong W (2001) Micropropagation of potato: evaluation of closed, diffusive and forced ventilation on growth and tuberization. Ann Bot 87:53–59
Acknowledgment
The authors are grateful to the Loyola College Management, Chennai for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: D. Duncan
Rights and permissions
About this article
Cite this article
Vinoth, A., Ravindhran, R. Reduced hyperhydricity in watermelon shoot cultures using silver ions. In Vitro Cell.Dev.Biol.-Plant 51, 258–264 (2015). https://doi.org/10.1007/s11627-015-9698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9698-5