Summary
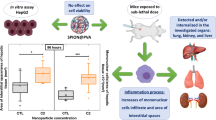
Biodistribution and toxicity assessment are critical for safe clinical use of newly developed medicines. Superparamagnetic iron oxide nanoparticles (SPION) are effective carriers for targeted drug delivery. This study aimed to examine the toxicity and biodistribution of SPION coated with polyethylenimine (PEI) (SPION-PEI) designed for small interfering RNA (siRNA) delivery both in vitro and in vivo. SPION-PEI/siRNA complexes were prepared at different weight ratios. Cytotoxic effects of SPION-PEI/siRNA on HSC-T6 cell viability were determined by using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). Rats were divided into three groups: a control group, a normal-saline group and a SPION-PEI/siRNA group. After a single intravenous injection, in vivo nanoparticle biodistribution and accumulation were evaluated by Prussian blue staining in the heart, liver, spleen, lung and kidney 8 h, 24 h, and 7 days after the injection. Their distribution was histologically studied at the three time points by measuring ironpositive areas (μm2) in organ sections stained with Prussian blue. The same organs were analyzed by H&E staining for any possible histopathological changes. Furthermore, biochemical indexes such as alanine amino transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN) and creatinine (CREA) were also assessed at all experimental time points. Electrophoresis exhibited that the SPION-PEI could retard siRNA altogether at weight ratios above 4. MTT assay showed that SPION-PEI loaded with siRNA had low cytotoxicity. In vivo study revealed that the liver and spleen were the major sites of SPION-PEI/siRNA deposition. The iron content was significantly increased in the liver and spleen, peaking 24 h after intravenous injection and then declining gradually. No evidence was found of irreversible histopathological damage to any of the organs tested. These results suggested that most SPION-PEI/siRNA complexes were distributed in the liver and spleen, which might be the target organs of SPION-PEI/siRNA complexes. SPIONPEI/siRNA may serve as in vivo carrier for biomedical medicines.
Similar content being viewed by others
References
Davidson BL, McCray PB. Current prospects for RNA interference-based therapies. Nat Rev Genet, 2011,12(5):329–340
Ungureanu BS, Teodorescu CM, Săfoiu A. Magnetic nanoparticles for hepatocellular carcinoma diagnosis and therapy. J Gastrointestin Liver Dis, 2016,25(3):375–383
Pecot CV, Calin GA, Coleman RL, et al. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer, 2011,11(1):59–67
Barreto JA, Malley WO, Kubeil M, et al. Nanomaterials: applications in cancer imaging and therapy. Adv Mater, 2011, 23(12): H18–H40
Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev, 2008,60(15):1638–1649
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B-Biointerfaces, 2010,75(1):1–18
Estelrich J, Sanchez-Martin MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int J Nanomed, 2015,10:1727–1741
Estelrich J, Escribano E, Queralt J, et al. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci, 2015,16(4):8070–8101
Sawdon A, Weydemeyer E, Peng CA. Antitumor therapy using nanomaterial-mediated thermolysis. J Biomed Nanotechnol, 2014,10(9):1894–1917
Liu G, Xie J, Zhang F, et al. N-Alkyl-PEI-Functionalized iron oxide nanoclusters for efficient siRNA delivery. Small, 2011,7(19):2742–2749
Kievit FM, Veiseh O, Fang C, et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano, 2010,4(8):4587–4594
Mok H, Veiseh O, Fang C, et al. pH-Sensitive siRNA nanovector for targeted gene silencing and cytotoxic effect in cancer cells. Mol Pharm, 2010,7(6):1930–1939
Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res, 2011,44(10):853–862
Chouly C, Pouliquen D, Lucet I, et al. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul, 1996,13(3):245–255
Gu L, Fang RH, Sailor MJ, et al. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano, 2012,6(6):4947–4954
Veiseh O, Kievit FM, Liu V, et al. In vivo safety evaluation of polyarginine coated magnetic nanovectors. Mol Pharm, 2013,10(11):4099–4106
Bellusci M, Barbera AL, Padella F, et al. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int J Nanomedicine, 2014,9(1):1919–1929
Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine, 2012,7:3445–3471
Chen J, Zhu S, Tong LQ, et al. Superparamagnetic iron oxide nanoparticles mediated 131I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. BMC Cancer, 2014,14(1):114
Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol, 1975,98:503–517
Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethyleneimine) and its derivatives. J Gene Med, 2005,7(8):992–1009
DeJong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed, 2008,3(2):133
Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine, 2008,3(5):703–717
Tsuchiya K, Nitta N, Sonoda A, et al. Histological study of the biodynamics of iron oxide nanoparticles with different diameters. Int J Nanomedicine, 2011,6:1587–1594
Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev, 2010,62(3):284–304
Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng, 1999,1(1):241–263
Tapan KJ, Morales MA, Leslie-Pelecky DL, et al. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm, 2007,5(2): 316–327
Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release, 2001, 74(1-3):7–25
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Nos. 81402640, 81502816), the Natural Science Foundation of Hubei Province (No. 2014CFB406), the Health and Family Planning Commission of Wuhan City (No. WX15B23) and Training Plan for Young and Middleaged Backbone Talents in Wuhan [No. 2014 (77)].
Rights and permissions
About this article
Cite this article
Yu, Q., Xiong, Xq., Zhao, L. et al. Biodistribution and Toxicity Assessment of Superparamagnetic Iron Oxide Nanoparticles In Vitro and In Vivo. CURR MED SCI 38, 1096–1102 (2018). https://doi.org/10.1007/s11596-018-1989-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1989-8