Abstract
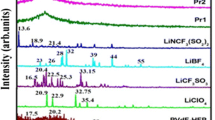
Solid polymer electrolytes based on high molecular weight poly(ethylene oxide) (PEO) complexed with lithium difluoro(oxalato)borate (LiDFOB) salt in various EO:Li molar ratios from 30:1 to 8:1 were prepared by using solution casting technique. Ion–polymer interaction, structural, thermal, and ionic conductivity studies have been reported by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), polarized optical microscopy (POM), differential scanning calorimeter (DSC), and impedance analysis. FTIR spectral studies suggested that the interaction of Li+ cations with the ether oxygen of PEO, where a triple peak broad band centered at 1105 cm−1, corresponds to C–O–C stretching and extreme deformation occurs. XRD, POM, and DSC indicated that the inclusion of LiDFOB salt could reduce the crystallinity of PEO. The melting temperature of PEO shifted to lower temperature side by the addition of LiDFOB. The glass transition temperature obtained for the system 10:1 was −38.2 °C. An increase in the ionic conductivity from 3.95 × 10−9 to 3.18 × 10−5 S/cm at room temperature (23 °C) was obtained through the addition of LiDFOB to a high molecular weight PEO. In addition, the ionic conductivity of the polymer electrolyte films followed an Arrhenius relation, and the activation energy decreased with increasing LiDFOB concentration.













Similar content being viewed by others
References
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Giddey S, Badwal SPS (2013) Polymer electrolyte membrane fuel cell as a hydrogen flow rate monitoring device. Ionics 19:523–528
Singh PK, Kim KW, Rhee HW (2009) Quantum dot doped solid polymer electrolytes for device application. Electrochem Commun 11:1247–1250
Sequeira CAC, Santos DMF (2010) Polymer electrolytes: fundamentals and applications. Woodhead Publishing Ltd., Cambridge, p 431
Dam T, Karan NK, Thomas R, Pradhan DK, Katiyar RS (2014) Observation of ionic transport and ion-coordinated segmental motions in composite (polymer-salt-clay) solid polymer electrolyte. Ionics. doi:10.1007/s11581-014-1181-5
Yoshino A (2012) The birth of the lithium-ion battery. Angew Chem Int Ed 51:5798–5800
Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10:439–448
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Mao G, Perea RF, Howells WS, Price DL, Saboungi ML (2000) Relaxation in polymer electrolytes on the nanosecond timescale. Nature 405:163–165
Reddy MJ, Chu PP (2002) Optical microscopy and conductivity of poly(ethylene oxide) complexed with KI salt. Electrochim Acta 47:1189–1196
Andersson AM, Edstrom K (2001) chemical composition and morphology of the elevated temperature SEI on graphite. J Electrochem Soc 148:A1100–A1109
Pasquier AD, Blyr A, Courjal P, Armatucci G, Gerand B, Tarascon JM (1999) Mechanism for limited 55 °C storage performance of Li1.05Mn1.95O4. J Electrochem Soc 146:428–436
Jow TR, Xu K, Ding MS, Zhang SS, Allen JL, Amine K (2004) LiBOB-based electrolytes for Li-ion batteries for transportation applications. J Electrochem Soc 151:A1702–A1706
Aravindan V, Vickraman P, Krishnaraj K (2009) Li+ ion conduction in TiO2 filled polyvinylidenefluoride-co-hexafluoropropylene based novel nanocomposite polymer electrolyte membranes with LiDFOB. Curr Appl Phys 9:1474–1479
Zhang SS, Xu K, Jow TR (2002) Study of LiBF4 as an electrolyte salt for a Li-ion battery. J Electrochem Soc 149:A586–A590
Zhou L, Li W, Xu M, Lucht B (2011) Investigation of the disproportionation reactions and equilibrium of lithium difluoro(oxalato) borate (LiDFOB). Electrochem Solid-State Lett 14:A161–A164
Zhang SS (2006) An unique lithium salt for the improved electrolyte of Li-ion battery. Electrochem Commun 8:1423–1428
Jow TR, Zhang SS, Xu K (2007) Additive for enhancing the performance of electrochemical cells. U.S. Pat 7172834
Zhang SS (2007) Electrochemical study of the formation of a solid electrolyte interface on graphite in a LiBC2O4F2-based electrolyte. J Power Sources 163:713–718
Chen Z, Liu J, Amine K (2007) Lithium difluoro(oxalate)borate as salt for lithium-ion batteries. Electrochem Solid-State Lett 10:A45–A47
Liu J, Chen Z, Busking S, Amine K (2007) Lithium difluoro(oxalate)borate as a functional additive for lithium-ion batteries. Electrochem Commun 9:475–479
Bruce PG, Vincent CA (1993) Polymer electrolytes. J Chem Soc Faraday Trans 89:3187–3203
Dey SA, Karan S, De SK (2009) Effect of nanofillers on thermal and transport properties of potassium iodide–polyethylene oxide solid polymer electrolyte. Solid State Commun 149:1282–1287
Takahashi Y, Tadokoro H (1973) Structural studies of polyethers, (-(CH2)m-O-)n. X. Crystal structure of poly(ethylene oxide). Macromolecules 6:672–675
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37:1371–1376
Appetecchi GB, Henderson W, Villano P, Berrettoni M, Passerini S (2001) PEO-LiN(SO2CF2CF3)2 polymer electrolytes: 1. XRD, DSC, and ionic conductivity characterization. J Electrochem Soc 148:A1171–A1178
Polu AR, Kumar R, Joshi GM (2014) Effect of zinc salt on transport, structural, and thermal properties of PEG-based polymer electrolytes for battery application. Ionics 20:675–679
Han SD, Allen JL, Jonsson E, Johansson P, McOwen DW, Boyle PD, Henderson WA (2013) Solvate structures and computational/spectroscopic characterization of lithium difluoro(oxalato)borate (LiDFOB) electrolytes. J Phys Chem C 117:5521–5531
Holomb R, Xu W, Markusson H, Johansson P, Jacobsson P (2006) Vibrational spectroscopy and ab initio studies of Lithium bis(oxalato)borate (LiBOB) in different solvents. J Phys Chem A 110:11467–11472
Kumar KK, Ravi M, Pavani Y, Bhavani S, Sharma AK, Rao VVRN (2014) Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J Membr Sci 454:200–211
Tang Z, Wang J, Chena Q, He W, Shen C, Mao XX, Zhang J (2007) A novel PEO-based composite polymer electrolyte with absorptive glass mat for Li-ion batteries. Electrochim Acta 52:6638–6643
Rocco AM, Bielschowsky CE, Pereira RP (2003) Blends of poly(ethylene oxide) and poly(4-vinylphenol-co-2-hydroxyethyl methacrylate): thermal analysis, morphological behaviour and specific interactions. Polymer 44:361–368
Rocco AM, Fonseca CP, Pereira RP (2002) A polymeric solid electrolyte based on a binary blend of poly(ethylene oxide), poly(methyl vinyl ether-maleic acid) and LiClO4. Polymer 43:3601–3609
Frech R, Chintapalli S, Bruce PG, Vincent CA (1999) Crystalline and amorphous phases in the poly(ethylene oxide)-LiCF3SO3 system. Macromolecules 32:808–813
Ibrahim S, Yassin MM, Ahmad R, Johan MR (2011) Effects of various LiPF6 salt concentrations on PEO-based solid polymer electrolytes. Ionics 17:399–405
Rajendran S, Kannan R, Mahendran O (2001) Ionic conductivity studies in poly(methylmethacrylate)–polyethlene oxide hybrid polymer electrolytes with lithium salts. J Power Sources 96:406–410
Ramesh S, Yuen TF, Shen CJ (2008) Conductivity and FTIR studies on PEO–LiX [X: CF3SO3 −, SO4 2−] polymer electrolytes. Spectrochim Acta A 69:670–675
Sundar M, Selladurai S (2006) Effect of fillers on magnesium–poly(ethylene oxide) solid polymer electrolyte. Ionics 12:281–286
Reddy MJ, Chu PP (2002) Ion pair formation and its effect in PEO:Mg solid polymer electrolyte system. J Power Sources 109:340–346
Yang Y, Huo H (2013) Investigation of structures of PEO-MgCl2 based solid polymer electrolytes. J Polym Sci B Polym Phys 51:1162–1174
Wen SJ, Richardson TJ, Ghantous DI, Striebel KA, Ross PN, Cairns EJ (1996) FTIR characterization of PEO + LiN(CF3SO2)2 electrolytes. J Electroanal Chem 408:113–118
Noor IS, Majid SR, Arof AK (2013) Poly(vinyl alcohol)–LiBOB complexes for lithium–air cells. Electrochim Acta 102:149–160
Preechatiwong W, Schultz JM (1996) Electrical-conductivity of poly(ethylene oxide)-alkali metal salt systems and effects of mixed salts and mixed molecular-weights. Polymer 37:5109–5116
Samir MASA, Alloin F, Gorecki W, Sanchez JY, Dufresne A (2004) Nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose nanocrystals. J Phys Chem B 108:10845–10852
Bhide A, Hariharan K (2007) Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur Polym J 43:4253–4270
Kumar Y, Hashmi SA, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolyte. Electrochim Acta 56:3864–3873
Mitra S, Kulkarni AR (2002) Electrical conductivity studies on the plasticised PEO–DBP–CdX (X=Cl; SO4) polymer electrolytes. Solid State Ionics 154:37–43
Lanfredi S, Saia PS, Lebullenger R, Hernandes AC (2002) Electric conductivity and relaxation in fluoride, fluorophosphate and phosphate glasses: analysis by impedance spectroscopy. Solid State Ionics 146:329–339
Ramya CS, Selvasekarapandian S, Hiran Kumar G, Savitha T, Angelo PC (2008) Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J Non-Cryst Solids 354:1494–1502
Chiodelli G, Ferloni P, Magistris A, Sanesi M (1988) Ionic conduction and thermal properties of poly(ethylene oxide)-lithium tetrafluoroborate films. Solid State Ionics 28–30:1009–1013
Wu X-L, Xin S, Seo H-H, Kim J, Guo Y-G, Lee J-S (2011) Enhanced Li+ conductivity in PEO-LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 186:1–6
Karan NK, Pradhan DK, Thomas R, Natesan B, Katiyar RS (2008) Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro- methane sulfonate (PEO–LiCF3SO3): ionic conductivity and dielectric relaxation. Solid State Ionics 179:689–696
Cho S, Cha W, Park HJ, Lee JM, Kim EB, Rhee HW, Jiangc Z, Strzalka J, Kim H (2013) Effects of siloxane nanoparticles on glass transition temperature and crystallization in PEO-LiPF6 polymer electrolytes. Synth Met 177:110–113
Polu AR, Kumar R, Rhee HW (2015) Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics 21:125–132
Shriver DF, Ratner MA (1988) Ion transport in solvent-free polymers. Chem Rev 88:109–124
Acknowledgments
This work was supported by Global Frontier R&D Program on Center for Multiscale Energy System funded by the National Research Foundation under the Ministry of Science, ICT & Future Planning, Korea (2011–0031570) and by the Korea Center for Artificial Photosynthesis (KCAP) located in Sogang University funded by the Minister of Science, ICT and Future Planning (MSIP) through the National Research Foundation of Korea (No. 2009–0093883) and also supported by the Human Resources Development program (No. 20114010203090) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean government Ministry of Trade, Industry and Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polu, A.R., Kim, D.K. & Rhee, HW. Poly(ethylene oxide)-lithium difluoro(oxalato)borate new solid polymer electrolytes: ion–polymer interaction, structural, thermal, and ionic conductivity studies. Ionics 21, 2771–2780 (2015). https://doi.org/10.1007/s11581-015-1474-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1474-3