Abstract
Background
Sarcopenia is a state of degenerative skeletal muscle wasting induced by cancer cachexia.
Objective
To evaluate the prognostic impact of changes in skeletal muscle mass (SMM) during first-line sunitinib therapy on oncological outcomes in metastatic renal cell carcinoma (mRCC).
Patients and Methods
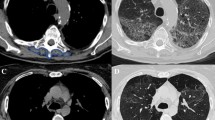
Sixty-nine patients were evaluated retrospectively. The skeletal muscle index (SMI) was calculated based on computed tomography images obtained before the initiation (pre-treatment SMI) and after two cycles of sunitinib treatment (post-treatment SMI). The change in SMM was evaluated based on the value of ΔSMI, which was calculated as [(posttreatment SMI – pretreatment SMI)/ pretreatment SMI] × 100. Oncological outcomes were compared between patients with ΔSMI <0 (SMM decrease) and ΔSMI ≥0 (SMM maintenance).
Results
A decrease in SMM was observed in 38 patients (55.1%). Progression-free survival (PFS) and overall survival (OS) after sunitinib therapy initiation were significantly shorter in patients with ΔSMI <0 than in those with ΔSMI ≥0 (median PFS: 9.53 vs. 28.4 months, p < 0.0001; OS: 19.8 vs. 52.6 months, p = 0.0001). ΔSMI was an independent predictive factor for PFS (HR 3.25, 95% CI 1.74–6.29, p = 0.0002) and OS (HR 4.53, 95% CI 2.15–10.5, p < 0.0001). The objective response rate was significantly lower in patients with ΔSMI <0 than in those with ΔSMI ≥0 (23.7% vs. 51.6%, p = 0.0164).
Conclusion
Decreased SMM during first-line sunitinib therapy can be an effective marker of outcome prediction for mRCC.





Similar content being viewed by others
References
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96.
Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 2018;268(1):58–69.
Hiraoka A, Otsuka Y, Kawasaki H, Izumoto H, Ueki H, Kitahata S, et al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(6):1271–6.
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–8.
Takamori S, Toyokawa G, Okamoto T, Shimokawa M, Kinoshita F, Kozuma Y, et al. Clinical impact and risk factors for skeletal muscle loss after complete resection of early non-small cell lung cancer. Ann Surg Oncol. 2018;25(5):1229–36.
Tsukioka T, Nishiyama N, Izumi N, Mizuguchi S, Komatsu H, Okada S, et al. Sarcopenia is a novel poor prognostic factor in male patients with pathological stage I non-small cell lung cancer. Jpn J Clin Oncol. 2017;47(4):363–8.
Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120(18):2910–8.
Mayr R, Gierth M, Zeman F, Reiffen M, Seeger P, Wezel F, et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. 2018;9(3):505–13.
Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: a retrospective multi-institution study. Int J Clin Oncol. 2017;22(1):136–44.
Psutka SP, Boorjian SA, Moynagh MR, Schmit GD, Costello BA, Thompson RH, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. 2016;195(2):270–6.
Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Investig New Drugs. 2017;35(4):436–41.
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–6.
Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23(3):658–65.
Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33.
Yoshikawa T, Takano M, Miyamoto M, Yajima I, Shimizu Y, Aizawa Y, et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother Pharmacol. 2017;80(3):555–61.
Taguchi S, Akamatsu N, Nakagawa T, Gonoi W, Kanatani A, Miyazaki H, et al. Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer. 2016;14(3):237–43.
Kacevska M, Robertson GR, Clarke SJ, Liddle C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Expert Opin Drug Metab Toxicol. 2008;4(2):137–49.
Cushen SJ, Power DG, Teo MY, MacEneaney P, Maher MM, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with Sunitinib. Am J Clin Oncol. 2017;40(1):47–52.
Ishihara H, Kondo T, Omae K, Takagi T, Iizuka J, Kobayashi H, et al. Sarcopenia and the modified Glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line Sunitinib treatment. Target Oncol. 2016;11(5):605–17.
Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl. Compr Cancer Netw. 2017;15(6):804–34.
Daly LE, Power DG, O’Reilly A, Donnellan P, Cushen SJ, O’Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–7.
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. 2018;9(2):315–25.
Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7(4):458–66.
Fukushima H, Kataoka M, Nakanishi Y, Sakamoto K, Takemura K, Suzuki H, et al. Posttherapeutic skeletal muscle mass recovery predicts favorable prognosis in patients with advanced urothelial carcinoma receiving first-line platinum-based chemotherapy. Urol Oncol. 2018;36(4):156.e9–156.e16.
Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal Cancer surgery. Ann Surg Oncol. 2015;22(13):4445–52.
Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–60.
Ikeda T, Ishihara H, Takagi T, Kondo T, Yoshida K, Iizuka J, et al. Prognostic impact of the components of progressive disease on survival after first-line tyrosine kinase inhibitor therapy for metastatic renal cell carcinoma. Target Oncol. 2018;13(3):379–87.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47.
Iwamoto K, Ishihara H, Takagi T, Kondo T, Yoshida K, Iizuka J, et al. Evaluation of relative dose intensity during the early phase of first-line sunitinib treatment using a 2-week-on/1-week-off regimen for metastatic renal cell carcinoma. Med Oncol. 2018;35(6):78.
Kondo T, Takagi T, Kobayashi H, Iizuka J, Nozaki T, Hashimoto Y, et al. Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma--comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn J Clin Oncol. 2014;44(3):270–7.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Vera-Badillo FE, Templeton AJ, Duran I, Ocana A, de Gouveia P, Aneja P, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. 2015;67(4):740–9.
Kroeger N, Xie W, Lee JL, Bjarnason GA, Knox JJ, Mackenzie MJ, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the international mRCC database consortium criteria. Cancer. 2013;119(16):2999–3006.
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9.
Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8.
Beuselinck B, Vano YA, Oudard S, Wolter P, De Smet R, Depoorter L, et al. Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int. 2014;114(1):81–9.
Zhou L, Cai X, Liu Q, Jian ZY, Li H, Wang KJ. Prognostic role of C-reactive protein in urological cancers: a meta-analysis. Sci Rep. 2015;5:12733.
Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J, et al. Effect of systemic inflammation on survival in patients with metastatic renal cell carcinoma receiving second-line molecular-targeted therapy. Clin Genitourin Cancer. 2017;15(4):495–501.
Tanaka N, Mizuno R, Yasumizu Y, Ito K, Shirotake S, Masunaga A, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma treated with first-line and subsequent second-line targeted therapy: a proposal of the modified-IMDC risk model. Urol Oncol. 2017;35(2):39.e19–28.
Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011;6(7):e22538.
Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41.
Sala D, Sacco A. Signal transducer and activator of transcription 3 signaling as a potential target to treat muscle wasting diseases. Curr Opin Clin Nutr Metab Care. 2016;19(3):171–6.
Ma JF, Sanchez BJ, Hall DT, Tremblay AK, Di Marco S, Gallouzi IE. STAT3 promotes IFNgamma/TNFalpha-induced muscle wasting in an NF-kappaB-dependent and IL-6-independent manner. EMBO Mol Med. 2017;9(5):622–37.
Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62.
Miyake H, Matsushita Y, Watanabe H, Tamura K, Suzuki T, Motoyama D, et al. Significance of introduction of alternative dosing schedule for sunitinib during first-line treatment of patients with metastatic renal cell carcinoma. Med Oncol. 2018;35(10):133.
Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. 2015;26(11):2300–5.
Bjarnason GA, Khalil B, Hudson JM, Williams R, Milot LM, Atri M, et al. Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol. 2014;32(4):480–7.
Acknowledgements
The authors thank Nobuko Hata for providing secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Tsunenori Kondo received honoraria from Pfizer, Bayer, and Novartis. All other authors including Hiroki Ishihara, Toshio Takagi, Hironori Fukuda, Kazuhiko Yoshida, Junpei Iizuka, and Kazunari Tanabe declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Ishihara, H., Takagi, T., Kondo, T. et al. Effect of Changes in Skeletal Muscle Mass on Oncological Outcomes During First-Line Sunitinib Therapy for Metastatic Renal Cell Carcinoma. Targ Oncol 13, 745–755 (2018). https://doi.org/10.1007/s11523-018-0600-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-018-0600-3