Abstract
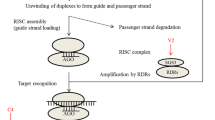
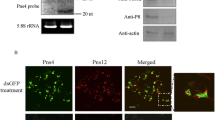
RNA silencing is a potent antiviral mechanism in plants and animals. As a counter-defense, many viruses studied to date encode one or more viral suppressors of RNA silencing (VSR). In the latter case, how different VSRs encoded by a virus function in silencing remains to be fully understood. We previously showed that the nonstructural protein Pns10 of a Phytoreovirus, Rice dwarf virus (RDV), functions as a VSR. Here we present evidence that another nonstructural protein, Pns11, also functions as a VSR. While Pns10 was localized in the cytoplasm, Pns11 was localized both in the nucleus and chloroplasts. Pns11 has two bipartite nuclear localization signals (NLSs), which were required for nuclear as well as chloroplastic localization. The NLSs were also required for the silencing activities of Pns11. This is the first report that multiple VSRs encoded by a virus are localized in different subcellular compartments, and that a viral protein can be targeted to both the nucleus and chloroplast. These findings may have broad significance in studying the subcellular targeting of VSRs and other viral proteins in viral-host interactions.
Similar content being viewed by others
References
Bédard, J., and Jarvis, P. (2005). Recognition and envelope translocation of chloroplast preproteins. J Exp Bot 56, 2287–2320.
Boualem, A., Dogimont, C., and Bendahmane, A. (2016). The battle for survival between viruses and their host plants. Curr Opin Virol 17, 32–38.
Cao, X., Zhou, P., Zhang, X., Zhu, S., Zhong, X., Xiao, Q., Ding, B., and Li, Y. (2005). Identification of an RNA silencing suppressor from a plant double-stranded RNA virus. J Virol 79, 13018–13027.
Caplan, J.L., Mamillapalli, P., Burch-Smith, T.M., Czymmek, K., and Dinesh-Kumar, S.P. (2008). Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132, 449–462.
Csorba, T., Kontra, L., and Burgyán, J. (2015). Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 479–480, 85–103.
Dempsey, D.M.A., Vlot, A.C., Wildermuth, M.C., and Klessig, D.F. (2011). Salicylic Acid biosynthesis and metabolism. Arabidopsis Book 9, e0156.
Ding, S.W., and Voinnet, O. (2007). Antiviral immunity directed by small RNAs. Cell 130, 413–426.
Dong, X., van Wezel, R., Stanley, J., and Hong, Y. (2003). Functional characterization of the nuclear localization signal for a suppressor of posttranscriptional gene silencing. J Virol 77, 7026–7033.
Du, Z., Chen, A., Chen, W., Liao, Q., Zhang, H., Bao, Y., Roossinck, M.J., and Carr, J.P. (2014). Nuclear-cytoplasmic partitioning of cucumber mosaic virus protein 2b determines the balance between its roles as a virulence determinant and an RNA-silencing suppressor. J Virol 88, 5228–5241.
Duan, C.G., Fang, Y.Y., Zhou, B.J., Zhao, J.H., Hou, W.N., Zhu, H., Ding, S.W., and Guo, H.S. (2012). Suppression of Arabidopsis ARGONAUTE1-Mediated Slicing, Transgene-Induced RNA Silencing, and DNA Methylation by Distinct Domains of the Cucumber mosaic virus 2b Protein. Plant Cell 24, 259–274.
Emanuelsson, O., Nielsen, H., and von Heijne, G. (1999). ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8, 978–984.
González, I., Martínez, L., Rakitina, D.V., Lewsey, M.G., Atencio, F.A., Llave, C., Kalinina, N.O., Carr, J.P., Palukaitis, P., and Canto, T. (2010). Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to RNA silencing suppressor activity. MPMI 23, 294–303.
González, I., Rakitina, D., Semashko, M., Taliansky, M., Praveen, S., Palukaitis, P., Carr, J.P., Kalinina, N., and Canto, T. (2012). RNA binding is more critical to the suppression of silencing function of Cucumber mosaic virus 2b protein than nuclear localization. RNA 18, 771–782.
Gopal, P., Pravin Kumar, P., Sinilal, B., Jose, J., Kasin Yadunandam, A., and Usha, R. (2007). Differential roles of C4 and βC1 in mediating suppression of post-transcriptional gene silencing: Evidence for transactivation by the C2 of Bhendi yellow vein mosaic virus, a monopartite begomovirus. Virus Res 123, 9–18.
Guo, Z., Li, Y., and Ding, S.W. (2019). Small RNA-based antimicrobial immunity. Nat Rev Immunol 19, 31–44.
Hamera, S., Song, X., Su, L., Chen, X., and Fang, R. (2012). Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant J 69, 104–115.
Hamilton, A., Voinnet, O., Chappell, L., and Baulcombe, D. (2002). Two classes of short interfering RNA in RNA silencing. EMBO J 21, 4671–4679.
Horsch RB, F.J., Hoffmann, N.L., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231.
Howard, E.A., Zupan, J.R., Citovsky, V., and Zambryski, P.C. (1992). The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: Implications for nuclear uptake of DNA in plant cells. Cell 68, 109–118.
Jelenska, J., Yao, N., Vinatzer, B.A., Wright, C.M., Brodsky, J.L., and Greenberg, J.T. (2007). A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol 17, 499–508.
Jiang, L., Wei, C.H., and Li, Y. (2012). Viral suppression of RNA silencing. Sci China Life Sci 55, 109–118.
Jin, L., Qin, Q., Wang, Y., Pu, Y., Liu, L., Wen, X., Ji, S., Wu, J., Wei, C., Ding, B., et al. (2016). Rice Dwarf Virus P2 protein hijacks auxin signaling by directly targeting the rice OsIAA10 protein, enhancing viral infection and disease development. PLoS Pathog 12, e1005847.
Kang, Z. Ultrastructure of plant pathogenic fungi. Beijing: China Science and Technology Press, 1996.
Li, G., Froehlich, J.E., Elowsky, C., Msanne, J., Ostosh, A.C., Zhang, C., Awada, T., and Alfano, J.R. (2014). Distinct Pseudomonas type-III effectors use a cleavable transit peptide to target chloroplasts. Plant J 77, 310–321.
Li, Y., Bao, Y.M., Wei, C.H., Kang, Z.S., Zhong, Y.W., Mao, P., Wu, G., Chen, Z.L., Schiemann, J., and Nelson, R.S. (2004). Rice dwarf phytoreovirus segment S6-encoded nonstructural protein has a cell-to-cell movement function. J Virol 78, 5382–5389.
Lu, R., Folimonov, A., Shintaku, M., Li, W.X., Falk, B.W., Dawson, W.O., and Ding, S.W. (2004). Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc Natl Acad Sci USA 101, 15742–15747.
Lucy, A.P., Guo, H.S., Li, W.S., and Ding, S.W. (2000). Suppression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J 19, 1672–1680.
Mandal, M.K., Chandra-Shekara, A.C., Jeong, R.D., Yu, K., Zhu, S., Chanda, B., Navarre, D., Kachroo, A., and Kachroo, P. (2012). Oleic acid-dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell 24, 1654–1674.
Mérai, Z., Kerényi, Z., Kertész, S., Magna, M., Lakatos, L., and Silhavy, D. (2006). Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J Virol 80, 5747–5756.
Padmanabhan, M.S., and Dinesh-Kumar, S.P. (2010). All hands on deck—the role of chloroplasts, endoplasmic reticulum, and the nucleus in driving plant innate immunity. MPMI 23, 1368–1380.
Suzuki, N., Sugawara, M., Kusano, T., Mori, H., and Matsuura, Y. (1994). Immunodetection of rice dwarf phytoreoviral proteins in both insect and plant hosts. Virology 202, 41–48.
Töpfer, R., Matzeit, V., Gronenborn, B., Schell, J., and Steinbiss, H.H. (1987). A set of plant expression vectors for transcriptional and translational fusions. Nucl Acids Res 15, 5890.
Voinnet, O., Lederer, C., and Baulcombe, D.C. (2000). A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103, 157–167.
Wang, J.W., and Qi, Y. (2018). Plant non-coding RNAs and epigenetics. Sci China Life Sci 61, 135–137.
Wang, X., Lu, J., Lao, K., Wang, S., Mo, X., Xu, X., Chen, X., and Mo, B. (2019). Increasing the efficiency of CRISPR/Cas9-based gene editing by suppressing RNAi in plants. Sci China Life Sci 62, 982–984.
Wasternack, C., and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111, 1021–1058.
Wei, T., Kikuchi, A., Suzuki, N., Shimizu, T., Hagiwara, K., Chen, H., and Omura, T. (2006a). Pns4 of rice dwarf virus is a phosphoprotein, is localized around the viroplasm matrix, and forms minitubules. Arch Virol 151, 1701–1712.
Wei, T., Zhang, C., Hou, X., Sanfaçon, H., and Wang, A. (2013). The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog 9, e1003378.
Wei, T., Kikuchi, A., Moriyasu, Y., Suzuki, N., Shimizu, T., Hagiwara, K., Chen, H., Takahashi, M., Ichiki-Uehara, T., and Omura, T. (2006b). The spread of Rice dwarf Virus among cells of its insect vector exploits virus-induced tubular structures. J Virol 80, 8593–8602.
Wei, T., Shimizu, T., Hagiwara, K., Kikuchi, A., Moriyasu, Y., Suzuki, N., Chen, H., and Omura, T. (2006c). Pns12 protein of Rice dwarf virus is essential for formation of viroplasms and nucleation of viral-assembly complexes. J General Virol 87, 429–438.
Xiang, Y., Kakani, K., Reade, R., Hui, E., and Rochon, D.A. (2006). A 38-amino-acid sequence encompassing the arm domain of the cucumber necrosis virus coat protein functions as a chloroplast transit peptide in infected plants. J Virol 80, 7952–7964.
Xu, H., Li, Y., Mao, Z., Li, Y., Wu, Z., Qu, L., An, C., Ming, X., Schiemann, J., Casper, R., et al. (1998). Rice dwarf phytoreovirus segment S11 encodes a nucleic acid binding protein. Virology 240, 267–272.
Yang, X., Zhou, H., and Zhou, X. (2019). Rock paper scissors: CRISPR/Cas9-mediated interference with geminiviruses in plants. Sci China Life Sci 62, 1389–1391.
Yang, Z., and Li, Y. (2018). Dissection of RNAi-based antiviral immunity in plants. Curr Opin Virol 32, 88–99.
Zhang, X., Yuan, Y.R., Pei, Y., Lin, S.S., Tuschl, T., Patel, D.J., and Chua, N.H. (2006). Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20, 3255–3268.
Zhao, S., Hong, W., Wu, J., Wang, Y., Ji, S., Zhu, S., Wei, C., Zhang, J., and Li, Y. (2017). A viral protein promotes host SAMS1 activity and ethylene production for the benefit of virus infection. eLife 6, e27529.
Zhou, F., Pu, Y., Wei, T., Wei, T., Liu, H., Deng, W., Wei, C., Ding, B., Omura, T., and Li, Y. (2007a). The P2 capsid protein of the nonenveloped rice dwarf phytoreovirus induces membrane fusion in insect host cells. Proc Natl Acad Sci USA 104, 19547–19552.
Zhou, F., Wu, G., Deng, W., Pu, Y., Wei, C., and Li, Y. (2007b). Interaction of rice dwarf virus outer capsid P8 protein with rice glycolate oxidase mediates relocalization of P8. FEBS Lett 581, 34–40.
Zhu, S., Gao, F., Cao, X., Chen, M., Ye, G., Wei, C., and Li, Y. (2005). The rice dwarf virus P2 protein interacts with ent-Kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol 139, 1935–1945.
Zurbriggen, M.D., Carrillo, N., Tognetti, V.B., Melzer, M., Peisker, M., Hause, B., and Hajirezaei, M.R. (2009). Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J 60, 962–973
Acknowledgements
We thank Prof.Biao Dingfor critical comments and substantial reading the manuscript, Prof. Shouwei Ding for valuable suggestions and providing plasmid pCamBia1300-Tav2b, Prof. David Baulcombe for providing 16c seeds. We also like to thank Dr.s Yiming Bao, Bo Ren, Qiuting Ren for their assistance. This work was supported by grants from CARS-01-06 to X.H.X., Transgenic Research Program (2016ZX08010001) and the National Natural Science Foundation of China (31530062) to Y.L.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Compliance and ethics
The author(s) declare that they have no conflict of interest.
Supporting Information
Figure S1 Schematic of two products encoded by the full-length segment S11 of RDV. Pns11 was used in this study.
Figure S2 Subcellular localization of transiently expressed Pns11-GFP in epidermal cells of N. benthamiana.
Figure S3 Pns11-GFP and various Pns11-GFP mutants were transiently expressed in BY2 cells.
The supporting information is available online at life.scichina.com and www.springerlink.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Gao, F., Zhao, S., Men, S. et al. A non-structural protein encoded by Rice Dwarf Virus targets to the nucleus and chloroplast and inhibits local RNA silencing. Sci. China Life Sci. 63, 1703–1713 (2020). https://doi.org/10.1007/s11427-019-1648-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1648-3