Abstract
The amphibian populations have faced a drastic decline over the past decades. This decline has been associated with the presence of contaminants in the environment, among other environmental stressors. The present study tested the responses following the exposure to lithium (2.5 mg L−1) and selenium (10μg L−1), both isolated and as a mixture, on the metabolic status of the tail muscle of premetamorphic American bullfrog (Lithobates catesbeianus) through the assessment of the total protein content, mobilization of glucose and triglycerides, and the activity of lactate dehydrogenase (LDH). The exposure followed a 21-day assay with two sampling periods (on the 7th and 21st day after the onset of exposure) to evaluate the effects over time. The group exposed to the mixture presented a statistically decreased LDH activity (P < 0.05) in both sampling periods. The presence of selenium elicited a statistically significant increase (P < 0.05) in the glucose mobilization after 7 days of exposure. After 21 days, the animals exposed to selenium presented levels of glucose mobilization comparable to the control group. The mobilization of glucose and triglycerides remained similar to the control group for the animals exposed to lithium and to the mixture in both periods of sampling (P > 0.05). The total protein content did not show any statistical difference in the treated groups throughout the experiment (P > 0.05). The presented results highlight the importance of the assessment of mixtures that can occur in the environment, since the combination of contaminants may elicit distinct toxicity compared with the effects triggered by the chemicals isolated.
Similar content being viewed by others
Introduction
The impact of the anthropogenic actions on the aquatic environment is widely known (Derraik 2002; Grenni et al. 2018; Murphy et al. 2012; Regnault et al. 2014; Trombulak and Frissell 2000). As a consequence, modifications in the composition of water have been noticed, i.e., synthetic compounds have been introduced, and natural elements have reached concentrations above the natural background (Cuoco et al. 2015; López-Pacheco et al. 2019). These modifications have been proved as an environmental problem and a challenge for regulatory purposes (Murphy et al. 2012). In an effort to regulate the presence of organic and inorganic contaminants in Brazilian waters, the Brazilian Environmental Council (CONAMA) through the resolution No. 357/2005 established safe levels for contaminants that, once in freshwaters, should be harmless to the aquatic environment. For lithium and selenium, CONAMA considers concentrations of 2.5mg L−1 and 10 μg L−1, respectively, acceptable for the protection of aquatic life (Brasil 2005).
Lithium (Li) occurs in the aquatic environment in the cationic form (Aral and Vecchio-Sadus 2008). This alkaline element has been employed in a wide range of activities, from its use in medicine as psychiatric drug to air treatment, ceramics, and glass production. Also, Li has been used as constituent of electronic devices, this latter representing the main trend in the demand for this alkaline metal, due to the growing market of rechargeable batteries employed in electronic tools, from cell phones to electric vehicles (Kszos and Stewart 2003; Eftekhari 2019; Wanger 2011; Jaskula 2020). Once in the aquatic environment, Li can exert toxic effects to freshwater animals, which have been described by studies using fish (Emery et al. 1981; Hamilton 1995; Kszos et al. 2003), amphibians (Pinto Vidal et al. 2021a, b), and other aquatic organisms (Anderson 1950; Kszos et al. 2003). Selenium (Se) is a chalcogen; this element is considered as an essential micronutrient to humans as well to other vertebrate and invertebrate species, but once its concentrations exceed a relatively low threshold, this element can cause toxicity (Anderson 2020; Kurokawa and Berry 2013; Stadtman 1974). Selenium is also employed in several commercial and industrial activities, ranging from glass manufacturing to its use in shampoos as antidandruff agent (Anderson 2020). Important sources of selenium in the environment are the combustion of fossil fuels and coal, irrigations of seleniferous soils, discharge from mining, and smelting activities (Lemly 1985; Maier and Knight 1994; Canton and Van Derveer 1997; Sappington 2002; Brandt et al. 2017). This chalcogen has been shown to bioaccumulate in amphibians and biomagnify through food webs, which may represent an ecotoxicological concern (Lemly 1985; Snodgrass et al. 2003; Snodgrass et al. 2004; Massé et al. 2016; Lanctôt et al. 2017a). Moreover, this element has been recognized as toxic to aquatic organisms, including fish (Hamilton 1995; Lemly 2018), and amphibians (Browne and Dumont 1980; Lanctôt et al. 2017a, b; Pinto Vidal et al. 2021a, b). Levels around 10 μg L−1 of selenium were associated to a widely known environmental disaster, which led to a drastic decline in the fish population including elimination of many species at the Belews Lake, North Carolina (Lemly 1985; Canton and Van Derveer 1997). The US Environmental Protection Agency (USEPA) recommend levels of total dissolved selenium from 1.5 μg L−1 for lentic and 3.1 μg L−1 for lotic systems (USEPA 2016), which shows that the Brazilian legislation is more permissive for the total dissolved selenium in Brazilian freshwaters.
Amphibians contribute widely to ecosystem services. These vertebrates play an important role in the control of pest outbreaks; they also contribute to the food web dynamics and to the cycling of nutrients in both aquatic and terrestrial environments. They also impact in the architecture of the environment through its digging behavior, which contributes to the modification of the soil bulk density, which also serve as habitat for other species (deMaynadier and Hunter 1995; Hocking and Babbitt 2014). Even though they have a high relevance for the ecosystem and for the human societies, these animals have been suffering an important decline over the past decades (Beebee and Griffiths 2005; Heatwole 2011; Alroy 2015). The major threats for the amphibian’s survival are represented by the destruction of the their habitats, diseases, the increasing incidence of UV-B irradiation, and pollution (Alroy 2015; Beebee and Griffiths 2005; Heatwole 2011; Salla et al. 2015; Welsh and Ollivier 1998). In tropical regions such as the South America, especially in Brazil, data from Alroy (2015) indicate that amphibians (anurans) are under endangerment.
Considering that the pollution has been described as one of the potential causes for the amphibian’s decline added to the fact that the current Brazilian environmental legislation does not consider the interaction among contaminants in order to establish safe levels for contaminants in water intended to the protection of aquatic life, the current study assessed the metabolic responses (total protein content, mobilization of glucose and triglycerides, and lactate dehydrogenase (LDH) activity) in the tail muscle of premetamorphic American bullfrog. The mobilization and storage of macronutrients are under strict regulation throughout the metamorphic development of amphibians’ species (Dodd and Dodd 1976), being the tail muscle critical in the contribution of nutrients to the metabolic adjustment required to the completion of the metamorphosis (Zhu et al. 2020).
The results presented in the current work consist of a subset of data from a single experiment which was projected to assess biomarkers indicative of metamorphic development (Pinto Vidal et al. 2021a), the hepatotoxic responses (Pinto-Vidal et al. 2021b), and the metabolic status of the tail muscle (herein presented), following the exposure to lithium and selenium, isolated and mixed, at concentrations considered safe by the Brazilian environmental legislation. The responses expressed by the exposed animals in the previous and the present study highlight the necessity for an update in the Brazilian Environmental legislation for the accepted levels of lithium and selenium in freshwaters.
Material and methods
Animal model
Premetamorphic (Etkin 1935; Denver et al. 2002) American bullfrog (Lithobates catesbeianus) at Gosner’s stage 25 (Gosner 1960) was employed in the current study because this specie has been well described in the literature and widely used for ecotoxicological studies (Brown and Cai 2007; Chagas et al. 2020; Dal-Medico et al. 2014; Franco-Belussi et al. 2020; Jones-Costa et al. 2018; Ossana et al. 2013; Ossana et al. 2010; Salla et al. 2015). The premetamorphic animals (n = 120), without any signs of structural abnormality or topic pathogenic infection, were obtained from a frog farm in the State of São Paulo (22°46′53.2″S 47°24′17.0″W). Upon that, the animals were carried to the Laboratory of Physiology Conservation (LaFisC), Federal University of São Carlos, Sorocaba, Brazil, in order to start the acclimation period.
Study design
Acclimation and exposure conditions
The study design was the same as described in Pinto-Vidal et al. (2021a, b), including the animals, once these data are part of Pinto-Vidal’s graduation research project. In brief, the animals were acclimated in two 80-L aquaria over 6 days under controlled conditions (Table 1), the feeding regime consisted in two offers of Sera Goldy® ration (Sera GmBH, Germany) ad libitum. All the conditions and feeding regime were the same for the acclimation and the exposure period.
Exposure design
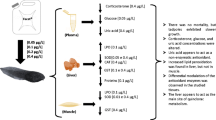
After the acclimation period, the animals were reassessed according to the Gosner’s staging, i.e., only animals at the Gosner’s stage 25 were employed in the exposure period. The chosen animals were individually exposed as described by Nunes et al. (2004), herein adapted to bullfrog tadpoles. Four groups were prepared (n = 20), being the negative control group (CT); the LI group, exposed to a total concentration of 2.5 mg L−1 of lithium (LiCl) (CAS Number: 7447-41-8); the SE group, exposed to a total concentration of 10μg L−1 of selenium (Na2SeO3) (CAS Number: 10102-18-8); and the SELI group, exposed to both elements mixed at their aforementioned concentrations. These concentrations represent what is considered safe for the protection of aquatic life by the Brazilian environmental legislation (Brasil 2005). The experiment was carried out for 21 days; the sampling occurred in two occasions (on the 7th and 21st day), when half of the animals (n= 10) were euthanized providing the biological material for the analyses. During the experiment, the static-renewal system was utilized, i.e., the entire exposure medium was renewed every 72 h, and a new exposure with the nominal concentrations of the chemicals was performed. The physicochemical parameters were checked right before every water change. The euthanasia of the animals followed the rules of the American Veterinary Medical Association (AVMA 2020). I.e., a blunt force trauma was applied to the craniocaudal region. All the procedures regarding the use of vertebrates were previously approved by the University’s Animal Ethics and Experimentation Committee (CEUA # 1397170117) from the Federal University of São Carlos, Brazil.
Biomarkers
The use of biomarkers in biomonitoring is well established; these ecotoxicological tools have been useful to evaluate the health status of a given environment (Dalzochio et al. 2016), predicting early stages of environmental disturbances (Vasseur and Cossu-Leguille 2003). The biomarkers employed in the current study were assessed in the tadpole’s tail muscle, being:
-
Total protein content
-
Glucose mobilization
-
Triglyceride mobilization
-
Activity of the lactate dehydrogenase (LDH)
These biomarkers were chosen in order to provide a general status of the metabolic competence of the premetamorphic tadpoles exposed to the treatments.
Preparation of the samples
After euthanasia, the samples were obtained by dissection of the tail muscle at the tail-body junction. The epidermis along with the dorsal and ventral fin was removed, and the tail muscle was separated from the notochord, upon that, the samples were immediately transferred to preservation under −80°C. The samples were homogenized with phosphate-buffered saline (PBS) (1.37-M NaCl, 0.027-M KCl, 0.054-M Na2HPO4.7H2O) solution, pH 7.2, for biochemical analysis. During the homogenization, the samples were kept in ice. Upon that, the homogenates were centrifuged at 12.000 rpm (Universal Centrifuge, 320R, Hettich®, Andreas Hettich GmbH & Co, Germany), for 20 min at 4 °C. The supernatants were collected and separated into four subsets of samples to provide the material for the different assays. Afterwards, the samples were stored under −80°C until the measurements were carried out. All the readings were performed in triplicate using a 96-well microtiter plate.
Total protein content
To assess the total protein content, we performed the dye-binding method proposed by Bradford (1976), which is suitable for the determination of small amounts of proteins in the samples. In brief, the samples were incubated in a 96-well microtiter plate with the Bradford reagent, which produces a stable blue color able to be read at the wavelength of 595 nm, using a spectrophotometer (Synergy HTX Multi-Mode Reader, BioTek Instruments, Inc., USA). The calibration curve was prepared with serum bovine albumin. The results were expressed as mg L −1 ± SD.
Assessment of glucose (GLU) and triglyceride (TRI) mobilization
In order to assess the mobilization of glucose and triglycerides, the samples were incubated with proper reagents designed to detect either free glucose (Labtest Liquiform Kit n°133; Labtest Diagnósticos S.A., Brazil) or free triglycerides (Labtest Liquiform Kit n° 87 Labtest Diagnósticos S.A., Brazil) in the homogenate. The product of the reaction was read at the wavelength of 505 nm, using a spectrophotometer (Synergy HTX Multi-Mode Reader, BioTek Instruments, Inc., USA) in a 96-well microtiter plate. The standard curve was prepared with the manufacturer’s solution. The results were expressed as mg dL−1 ± SD.
Assessment of the lactate dehydrogenase (LDH) activity
The method employed for the assessment of the LDH was first proposed by Bergmeyer and Bernt (1974). Briefly, the samples were transferred into the reaction medium which consisted of sodium pyruvate 1mM and reduced nicotinamide adenine dinucleotide (NADH+H+) 0.14 mM in a potassium phosphate buffer solution 100 mM (pH 7.4). Eight kinetic readings were performed (every 40 s); then, the decay of NADH+H+ and the appearance of NAD+ (oxidized nicotinamide adenine dinucleotide) were assessed, which are directly linked to the LDH activity. The product of this reaction was read in a 96-well microplate at the wavelength of 340 nm (Synergy HTX Multi-Mode Reader, BioTek Instruments, Inc., USA). The extinction coefficient of NADH+H+ was 6.22 cm−1 μmol−1. The results were expressed as international units per milligram of protein per minute (UI per mg of protein −1) ± SD.
Statistical analyses
The obtained data from all the biomarkers were previously tested for normality and homoscedasticity using the Kolmogorov-Smirnov (P > 0.05) and the Brown-Forsythe (P > 0.05) tests, respectively, considering that the data attended the precepts for the use of parametric tests we submitted to the analysis of variance (one-way ANOVA). The Bonferroni method was used as a post hoc test with the level of significance (α) = 0.05. Outliers were removed prior the statistical tests, add to that two animals from LI and SELI groups which died during the 3rd week, which explains the reduced sample size in the biochemical analyses compared to the number of exposed animals. All analyses were carried out using GraphPad Prism 5 for Windows (GraphPad Software, La Jolla, California, EUA), except for the Brown-Forsythe test that was performed using Excel for Windows (Microsoft Corporation, Redmond, Washington, USA).
Results
Total protein content in the tail muscle
The treatments with the chemicals (isolated or mixed) did not show statistically significant difference between the exposed groups and the CT at day 7 (CT = 85.71 ± 15.42 mg mL−1; LI = 103.36 ± 14.35 mg mL−1; SE = 95.80 ± 16.01 mg mL−1; SELI = 93.00 ± 18.62 mg mL−1) (F3.28 = 1.624, P > 0.05). After 21 days of exposure, the same pattern was observed: CT = 91.06 ± 18.00 mg mL−1; LI = 76.84 ± 14.80 mg mL−1; SE = 73.06 ± 12.30 mg mL−1; SELI = 84.70 ± 14.95 mg mL−1 (F3. 23 = 1.943, P > 0.05) (Fig. 1).
Total protein content assessed in the tail muscle. The control group at day 7 (CT 7), n= 7; the LI group at day 7 (LI 7), n= 9; the SE group at day 7 (SE 7), n= 8; and the SELI group at day 7 (SELI 7), n= 8. The control group at day 21 (CT 21), n= 7; the LI group at day 21 (LI 21), n= 6; the SE group at day 21 (SE 21), n= 7; and SELI group at day 21 (SELI 21), n= 7. The results are expressed as mean ± SD
Mobilization of glucose in the tail muscle
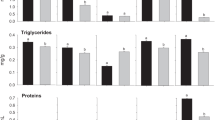
The analyses of variance showed that on the 7th day, there was a statistically significant difference among the groups (F3,31= 6.66, P < 0.05); the Bonferroni test revealed the statistical increase (P < 0.05) in the mobilization of glucose in the SE (2.93 ± 0.56 mg dL−1) group when compared to the CT group (2.07 ± 0.40 mg dL−1). The mobilization of glucose also presented a significant increase (P > 0.05) in the SE group on the 7th day when compared to that in the SELI group (2.10 ± 0.40 mg dL−1) at the same period. Meanwhile, the comparison between the LI (2.41 ± 0.42 mg dL−1) and the SELI groups (2.10 ± 0.40 mg dL−1) did not show any statistical difference when compared to the CT group or between each other (Fig. 2). On the 21st day, neither of the exposed groups (LI = 2.77 ± 0.51; SE = 2.83 ± 0.53 and SELI = 2.95 ± 0.56 mg dL−1) showed statistical difference when compared to the CT group (2.86 ± 0.49 mg dL−1) nor between the isolated chemicals and the mixture (F3.22 = 0.1325, P > 0.05).
Mobilization of glucose in the tail muscle. The control group at day 7 (CT 7), n= 8; the LI group at day 7 (LI 7), n= 10; the SE group at day 7 (SE 7), n= 8; and the SELI group at day 7 (SELI 7), n= 9. The control group at day 21 (CT 21), n= 7; the LI group at day 21 (LI 21), n= 6; the SE group at day 21 (SE 21), n= 6; and the SELI group at day 21 (SELI 21), n= 7. The results are expressed as mean ± SD. The (a) shows a statistical difference (P< 0.05) in comparison with the CT group at the same period. The (b) shows statistical difference when the group (isolated chemical) is compared with the mixture (the SELI group).
Mobilization of triglycerides in the tail muscle
The mobilization of the triglycerides (Fig. 3) in the tail muscle did not show any statistical difference between the CT (7.53 ± 1.30 mg dL−1) group and the exposed groups (LI = 7.91 ± 1.12; SE = 8.53 ± 1.40; SELI = 7.26 ± 0.90 mg dL−1) at the day 7 (F3.36 = 2.142, P > 0.05). After 21 days of exposure, the same pattern was observed (CT = 7.31 ± 1.01; LI = 7.93 ± 1.01; SE = 7.47 ± 1.08; SELI =7.51 ± 0.55 mg dL−1), i.e., no statistical difference (F3.29 = 0.9794, P > 0.05) among the groups.
Mobilization of triglycerides in the tail muscle. The control group at day 7 (CT 7), n= 10; the LI group at day 7 (LI 7), n= 10; the SE group at day 7 (SE 7), n= 10; and the SELI group at day 7 (SELI 7), n= 10. The control group at day 21 (CT 21), n= 9; the LI group at day 21 (LI 21), n= 8; the SE group at day 21 (SE 21), n= 8; and the SELI group at day 21 (SELI 21), n= 9. The results are expressed as mean ± SD
Assessment of the lactate dehydrogenase (LDH) activity
The assessment of the activity of LDH on the 7th day presented a statistical significance regarding the variance (F3, 28= 6.871, P = 0.0013). The Bonferroni test revealed the statistical relevant decrease in the activity of LDH from the SELI group (0.73 ± 0.13 UI mg−1) in comparison to the CT group (0.97 ± 0.18 UI mg−1) and with the SE group (1.04 ± 0.15 UI mg−1); meanwhile the LI group (0.91 ± 0.13 UI mg−1) remained statistically similar to the CT and the mixture (P > 0.05). At day 21, the variance was statistically relevant (F3.24 = 4.526, P = 0.0119), the Bonferroni test showed the decrease in the LDH activity on the 21st day (P < 0.05) for the SELI group (0.70 ± 0.12 UI mg−1) when compared to the CT group (1.03 ± 0.21 UI mg−1), whereas the LI (0.90 ± 0.15 UI mg−1) and SE groups (0.92 ± 0.15 UI mg−1) did not show statistically significant differences among either the control or the mixture group (P > 0.05) (Fig. 4).
Lactate dehydrogenase (LDH) activity in the tail muscle. The control group at day 7 (CT 7), n= 8; the LI group at day 7 (LI 7), n= 7; the SE group at day 7 (SE 7), n= 7; and the SELI group at day 7 (SELI 7), n= 7. The control group at day 21 (CT 21), n= 6; the LI group at day 21 (LI 21), n= 8; the SE group at day 21 (SE 21), n= 8; and the SELI group at day 21 (SELI 21), n= 6. The results are expressed as mean ± SD. The (a) shows a statistical difference (P < 0.05) in comparison with the CT group in the same period. The (b) shows a statistical difference when the group (isolated chemical) is compared to the mixture (the SELI group)
Discussion
During the larval stage of life, the anurans undergo several metabolic changes intended to prepare them for maturity. At every stage (premetamorphosis, prometamorphosis, and climax), specific adaptations in the physiology and structural organization, add to biochemical adjustments, have been described as critical for the development of a free swimming organism to a terrestrial air breathing animal (Brown and Cai 2007; Dodd and Dodd 1976; Zhu et al. 2020). Thus, considering the influence of every stage of life in the tadpole’s metabolic profile, we performed the assessment of the external characteristics following the Gosner’s (1960) criteria: all the animals during the whole experiment remained at the premetamorphic stage (unpublished data), which, according to Denver et al. (2002), ranges from the Gosner’s stage 25 to 35. Our data published elsewhere (Pinto Vidal et al. 2021a) shows that external development did not show statistical difference when the snout-vent length and the hind-limb length were used as a proxy to the evaluation of external development. This find can be explained by the longer time required by the chosen animal model to achieve its metamorphic climax, e.g., tail and gill resorption and development of the lugs (Miyata and Ose 2012), which for the Lithobates catesbeianus can take as long as 3 years (Brown and Cai 2007), under natural conditions. Furthermore, it is important to consider the mortality rate of 10% (2 animals) that occurred within the LI and SELI groups during the 3rd week; this mortality was preceded by a decrease in the feeding behavior and a disturbance in the external pattern coloration of referred animals (Pinto Vidal et al. 2021a); the possible cause of this mortality is still unknown.
The tail muscle is an important source of energetic compounds during the spontaneous metamorphic process. This organ establishes a metabolic network with the liver in order to provide macronutrients according to the developmental necessities (Zhu et al. 2020). In the current study, the total protein content in the tail muscle did not present any statistical difference among the groups. According to Yoshizato and Nakajima (1980), the relative protein content during the premetamorphosis and the climax stages does not present differences in quantity, but the quality of the amino acids differs in composition, being the tail of the premetamorphic animals rich in histidine and lysine, whereas at the climax, higher levels of glycine are expected (Yoshizato and Nakajima 1980).
The assessment of mobilization of glucose showed a statistical increase in the animals exposed to selenium after 7 days; however, after 21 days of exposure, the mobilization of glucose presented levels compared to those expressed by the animals in CT group. Studies carried out in fish have shown that the presence of organic selenium (selenomethionine) (2.8, 9.9, and 26.5 μg Se/g dry mass (dm)) was linked to decreased whole-body glycogen in fathead minnow (Pimephales promelas) (McPhee and Janz 2014). Regarding the effects on the carbohydrate metabolism triggered by inorganic selenium (sodium selenite), it was shown (Miller et al. 2007) that the acute (96h) exposure (1.8 and 3.6 mg L−1 Se) increased the plasma glucose in rainbow trout, which has increased along with the plasma cortisol, indicating a possible effect of the stress on the mobilization of glucose. However, a sub-chronic (30 days) exposure (0.36 mg L−1 Se) elicited an increase in the cortisol levels, without statistically significant effects on glucose levels (Miller et al. 2007). Conversely, other studies have shown that the exposure to selenium elicited no effects on the glucose metabolism in the whole-body Danio rerio (Massé et al. 2013) and muscle samples from Acipenser transmontanus (Patterson et al. 2017).
In the present study, the presence of inorganic selenium induced an increase in the mobilization of glucose after 7 days of exposure along with an increase (but not statistically relevant) in the LDH activity at the same period of sampling, which might indicate an increased demand for ATP generation in the tail muscle. Previous studies performed by Browne and Dumont (1980) showed that the larval Xenopus laevis exhibited an impairment in the muscle cell’s mitochondria (swelling and signs of degeneration) which could lead to impairment in the glicidic metabolism in the muscle. The SE group also presented statistically augmented levels of glucose mobilization when compared to the mixture at day 7. These findings indicate that the presence of lithium in the medium somehow antagonizes the effects of selenium, since the comparison between the SELI and CT groups did not differ statistically. After 21 days of exposure, the mobilization of glucose reached levels compared to the CT group, indicating an adaptation in the glicidic pathways.
Regarding the effects of selenium on triglyceride metabolism, Thomas et al. (2013) tested the influence of a range of concentrations of organic selenium (3.4, 9.8, and, 27.5 μg Se/g dm) in Danio rerio; for this animal model, the triglyceride content increased with the increasing in the Se concentration. Conversely, fathead minnow (Pimephales promelas) when fed with 5.4 μg Se/g dm presented a statistical reduction in the triglyceride content; however, in the same study, those animals fed with a wider range of Se concentrations (2.8, 9.9, and 26.5 μg Se/g dm) did not show any statistical differences in the whole-body triglyceride content (McPhee and Janz 2014). No differences in the triglyceride content were seen in the analyses in the adductor muscle of the adult Xenopus laevis (Massé et al. 2016). The current study did not find any statistically significant changes in the mobilization of triglycerides in the tail muscle of premetamorphic American bullfrog.
The LDH activity was reduced in the animals from the SELI group in both periods of sampling. Interestingly, on the 7th day, the animals from the SE group presented a statistically significant difference in this biomarker when compared to the SELI group, unveiling the distinct effect of the isolated chemicals when in a mixture. The inhibition of the LDH activity was seen in amphibians that were exposed to mercury (Hilmy et al. 1986) and copper (Chagas et al. 2020; Santos et al. 2013) at different stages of life, as well as in the muscle (Antognelli et al. 2003) and liver (Goel et al. 1985) of fish when exposed to copper and lithium, respectively. LDH is an important enzyme of anaerobic metabolism, being particularly important when a considerable amount of energy is rapidly required (Diamantino et al. 2001). The inhibition of the LDH may indicate an eventual impairment in the energetic metabolism, and this response has been linked to the stress imposed by the exposure to contaminants (Chagas et al. 2020; Santos et al. 2013).
The effects of exposure to mixtures of metal (loids) have been explored (Lefcort et al. 1998, Yologlu and Ozmen 2015; Carvalho et al. 2017, Chagas et al. 2020, Pinto Vidal et al. 2021a, b) in amphibians, and it represents an important field in the (eco) toxicological sciences; however, the knowledge on this subject needs to be extensively explored due to the potential toxicity of mixtures (Murphy et al. 2012). Assays that encompass more chemicals in the mixture need to be performed in order to build data on the effects of complex mixtures allowed by the Brazilian environmental legislation and with this information establish reliable and safe levels for the presence of multiple contaminants in the Brazilian waters.
Conclusion
The distinct responses elicited by the mixture when compared to the isolated chemicals ratify the necessity for testing the ecotoxicological profile of mixtures rather than isolated compounds in situations where several contaminants can coexist in the environmental compartments. The results herein presented highlighted the importance for an update in the Brazilian environmental legislation regarding the recommended levels of lithium and selenium in waters intended to protection of aquatic life.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alroy J (2015) Current extinction rates of reptiles and amphibians. Proc Natl Acad Sci U S A 112:13003–13008. https://doi.org/10.1073/pnas.1508681112
Anderson BG (1950) The apparent thresholds of toxicity to daphnia magna for chlorides of various metals when added to lake Erie water. Trans Am Fish Soc 78:96–113. https://doi.org/10.1577/1548-8659(1948)78[96:tatott]2.0.co;2
Anderson CS (2020) Selenium. U.S. Geological Survey (USGS), Mineral Commodity Summaries. p. 146-147. Avaiable in: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-selenium.pdf. Accessed 22.01.2021
Antognelli C, Romani R, Baldracchini F, De Santis A, Andreani G, Talesa V (2003) Different activity of glyoxalase system enzymes in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 142:297–305. https://doi.org/10.1016/S0009-2797(02)00124-2
Aral H, Vecchio-Sadus A (2008) Toxicity of lithium to humans and the environment-a literature review. Ecotoxicol Environ Saf 70:349–356. https://doi.org/10.1016/j.ecoenv.2008.02.026
AVMA (2020) American Veterinary Medical Association guidelines for the euthanasia of animals: 2020 edition. ESC CardioMed. https://doi.org/10.1093/med/9780198784906.003.0764
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125:271–285. https://doi.org/10.1016/j.biocon.2005.04.009
Bergmeyer HU, Bernt E (1974) Lactate dehydrogenase. UV-assay with pyruvate and NADH. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis. Academic, New York, pp 574–578
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bioanal 72:248–254. https://doi.org/10.1016/j.cj.2017.04.003
Brandt JE, Bernhardt ES, Dwyer GS, Di Giulio RT (2017) Selenium ecotoxicology in freshwater lakes receiving coal combustion residual effluents: a North Carolina example. Environ Sci Technol 51:2418–2426. https://doi.org/10.1021/acs.est.6b05353
Brasil (2005) Resolução CONAMA n° 357, de 17 de março de 2005. Conselho Nacional de Meio Ambiente [in Portuguse]. Available: http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=459. Accessed 20 Dec 2020
Brown DD, Cai L (2007) Amphibian metamorphosis. Dev Biol 306:20–33. https://doi.org/10.1016/j.ydbio.2007.03.021
Browne C, Dumont JN (1980) Cytotoxic effects of sodium selenite on tadpoles (Xenopus laevis). Arch Environ Contam Toxicol 9:181–191. https://doi.org/10.1007/BF01055373
Canton SP, Van Derveer WD (1997) Selenium toxicity to aquatic life: an argument for sediment-based water quality criteria. Environ Toxicol Chem 16:1255–1259. https://doi.org/10.1897/1551-5028(1997)016<1255:STTALA>2.3.CO;2
Carvalho CS, Utsunomiya HSM, Pasquoto T, Lima R, Costa MJ, Fernandes MN (2017) Blood cell responses and metallothionein in the liver, kidney and muscles of bullfrog tadpoles, Lithobates catesbeianus, following exposure to different metals. Environ Pollut 221:445–452. https://doi.org/10.1016/j.envpol.2016.12.012
Chagas BRC, Utsunomiya HSM, Fernandes MN, Carvalho CS (2020) Metabolic responses in bullfrog, Lithobates catesbeianus after exposure to zinc, copper and cadmium. Comp Biochem Physiol Part C Toxicol Pharmacol 233:108768. https://doi.org/10.1016/j.cbpc.2020.108768
Cuoco E, Darrah TH, Buono G, Verrengia G, De Francesco S, Eymold WK, Tedesco D (2015) Inorganic contaminants from diffuse pollution in shallow groundwater of the Campanian Plain (Southern Italy). Implications for geochemical survey. Environ Monit Assess 187(2):46. https://doi.org/10.1007/s10661-015-4307-y
Dal-Medico SE, Rissoli RZ, Gamero FU, Victório JA, Salla RF, Abdalla FC, Silva-Zacarin ECM, Carvalho CS, Costa MJ (2014) Negative impact of a cadmium concentration considered environmentally safe in Brazil on the cardiac performance of bullfrog tadpoles. Ecotoxicol Environ Saf 104:168–174. https://doi.org/10.1016/j.ecoenv.2014.03.003
Dalzochio T, Rodrigues GZP, Petry IE, Gehlen G, da Silva LB (2016) The use of biomarkers to assess the health of aquatic ecosystems in Brazil: a review. Int Aquat Res 8:283–298. https://doi.org/10.1007/s40071-016-0147-9
deMaynadier PG, Hunter ML Jr (1995) The relationship between forest management and amphibian ecology: a review of the North American literature. Environ Rev 3(3-4):230–261. https://doi.org/10.1139/a95-012
Denver RJ, Boorse GC, Glennemeier KA (2002) Endocrinology of complex life cycles: amphibians. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R (eds) Hormones, Brain and Behavior, vol 2. Academic Press, Inc., San Diego, pp 469–513
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Diamantino TC, Almeida E, Soares AMVM, Guilhermino L (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45:553–560. https://doi.org/10.1016/S0045-6535(01)00029-7
Dodd MHI, Dodd JM (1976) The biology of metamorphosis, physiology of the amphibia. Academic Press, Inc., San Diego. https://doi.org/10.1016/b978-0-12-455403-0.50015-3
Eftekhari A (2019) Lithium batteries for electric vehicles: from economy to research strategy. ACS Sustain Chem Eng 7:5602–5613. https://doi.org/10.1021/acssuschemeng.8b01494
Emery R, Klopfer DC, Skalski JR (1981) The incipient toxicity of lithium to freshwater organisms representing a salmo-nid habitat. PNL-3640, UC-11. Pacific Northwest Laboratory, Richland 364 p
Etkin W (1935) The mechanisms of anuran metamorphosis. I. Thyroxine concentrations and the metamorphic pattern. J Exp Zool 71:317–340
Franco-Belussi L, Jones-Costa M, Salla RF, Souza BFS, Pinto-Vidal FA, Oliveira CR, Silva-Zacarin ECM, Abdalla FC, Duarte ICS, De Oliveira C (2020) Hepatotoxicity of the anionic surfactant linear alkylbenzene sulphonate (LAS) in bullfrog tadpoles. Chemosphere:129014. https://doi.org/10.1016/j.chemosphere.2020.129014
Goel KA, Sharma SD, Maya (1985) Biochemical and enzymological changes in liver and kidney of Clarias batrachus following lithium intoxication. Arh Hig Rada Toksikol 36(3):249–253
Gosner KL (1960) Herpetologists “League a simplified table for staging anuran embryos and larvae with notes on identification author(s): Kenneth L. Gosner Published by: Allen Press on behalf of the Herpetologists” League Stable URL: http://www.jstor.org/stable/389. Herpetologica 16:183–190
Grenni P, Ancona V, Barra Caracciolo A (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39. https://doi.org/10.1016/j.microc.2017.02.006
Hamilton SJ (1995) Hazard assessment of inorganics to three endangered fish in the Green River, Utah. Ecotoxicol Environ Saf 30:134–142. https://doi.org/10.1006/eesa.1995.1017
Heatwole H (2011) Worldwide decline and extinction of amphibians. Balanc Nat Hum Impact 9781107019, pp 259–278. https://doi.org/10.1017/CBO9781139095075.025
Hilmy AA, El-Domaity N, Daabees AY (1986) Effect of ethylenediaminetetraacetic acid (EDTA) on acute mercury poisoning of toad. Comp Biochem Physiol 85:253–254. https://doi.org/10.1016/0742-8413(86)90083-6
Hocking DJ, Babbitt KJ (2014) Amphibian contributions to ecosystem services. Herpetol Conserv Biol 9(1):1–17
Jaskula BW (2020) Lithium. U.S. Geological Survey (USGS), Mineral Commodity Summaries. p. 98-99. Avaiable in: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-lithium.pdf. Accessed 22.01.2021
Jones-Costa M, Franco-Belussi L, Vidal FAP, Gongora NP, Castanho LM, dos Santos Carvalho C, Silva-Zacarin ECM, Abdalla FC, Duarte ICS, De Oliveira C, de Oliveira CR, Salla RF (2018) Cardiac biomarkers as sensitive tools to evaluate the impact of xenobiotics on amphibians: the effects of anionic surfactant linear alkylbenzene sulfonate (LAS). Ecotoxicol Environ Saf 151:184–190. https://doi.org/10.1016/j.ecoenv.2018.01.022
Kszos LA, Beauchamp JJ, Stewart AJ (2003) Toxicity of lithium to three freshwater organisms and the antagonistic effect of sodium. Ecotoxicology 12:427–437. https://doi.org/10.1023/A:1026160323594
Kszos LA, Stewart AJ (2003) Review of lithium in the aquatic environment: distribution in the United States, toxicity and case example of groundwater contamination. Ecotoxicology 12:439–447. https://doi.org/10.1023/A:1026112507664
Kurokawa S, Berry MJ (2013) Selenium. Role of the essential metalloid in health, Metal Ions in Life Sciences. https://doi.org/10.1007/978-94-007-7500-8-16
Lanctôt CM, Cresswell T, Callaghan PD, Melvin SD (2017a) Bioaccumulation and biodistribution of selenium in metamorphosing tadpoles. Environ Sci Technol 51:5764–5773. https://doi.org/10.1021/acs.est.7b00300
Lanctôt CM, Cresswell T, Melvin SD (2017b) Uptake and tissue distributions of cadmium, selenium and zinc in striped marsh frog tadpoles exposed during early post-embryonic development. Ecotoxicol Environ Saf 144:291–299. https://doi.org/10.1016/j.ecoenv.2017.06.047
Lefcort H, Meguire RA, Wilson LH, Ettinger WF (1998) Heavy metals alter the survival, growth, metamorphosis, and antipredatory behavior of Columbia spotted frog (Rana luteiventris) tadpoles. Arch Environ Contam Toxicol 35(3):447–456. https://doi.org/10.1007/s002449900401
Lemly AD (2018) Selenium poisoning of fish by coal ash wastewater in Herrington Lake, Kentucky. Ecotoxicol Environ Saf 150:49–53. https://doi.org/10.1016/j.ecoenv.2017.12.013
Lemly AD (1985) Toxicology of selenium in a freshwater reservoir: implications for environmental hazard evaluation and safety. Ecotoxicol Environ Saf 10:314–338. https://doi.org/10.1016/0147-6513(85)90079-X
López-Pacheco IY, Silva-Núñez A, Salinas-Salazar C, Arévalo-Gallegos A, Lizarazo-Holguin LA, Barceló D, Iqbal HMN, Parra-Saldívar R (2019) Anthropogenic contaminants of high concern: existence in water resources and their adverse effects. Sci Total Environ 690:1068–1088. https://doi.org/10.1016/j.scitotenv.2019.07.052
Maier KJ, Knight AW (1994) Ecotoxicology of selenium in freshwater systems. Rev Environ Contam Toxicol 134:31–48. https://doi.org/10.1007/978-1-4684-7068-0_2
Massé AJ, Muscatello JR, Janz DM (2016) Effects of elevated in ovo selenium exposure on late stage development of Xenopus laevis tadpoles. Bull Environ Contam Toxicol 97(4):463–468. https://doi.org/10.1007/s00128-016-1884-6
Massé AJ, Thomas JK, Janz DM (2013) Reduced swim performance and aerobic capacity in adult zebrafish exposed to waterborne selenite. Comp Biochem Physiol Part C Toxicol Pharmacol 157(3):266–271. https://doi.org/10.1016/j.cbpc.2012.12.004
McPhee DL, Janz DM (2014) Dietary selenomethionine exposure alters swimming performance, metabolic capacity and energy homeostasis in juvenile fathead minnow. Aquat Toxicol 155:91–100. https://doi.org/10.1016/j.aquatox.2014.06.012
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol 83(4):263–271. https://doi.org/10.1016/j.aquatox.2007.05.001
Miyata K, Ose K (2012) Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J Toxicol Pathol 25(1):1–9. https://doi.org/10.1293/tox.25.1
Murphy EA, Post GB, Buckley BT, Lippincott RL, Robson MG (2012) Future challenges to protecting public health from drinking-water contaminants. Annu Rev Public Health 33(1):209–224. https://doi.org/10.1146/annurev-publhealth-031811-124506
Nunes B, Carvalho F, Guilhermino L (2004) Acute and chronic effects of clofibrate and clofibric acid on the enzymes acetylcholinesterase, lactate dehydrogenase and catalase of the mosquitofish, Gambusia holbrooki. Chemosphere 57:1581–1589
Ossana NA, Castañé PM, Poletta GL, Mudry MD, Salibián A (2010) Toxicity of waterborne copper in premetamorphic tadpoles of lithobates catesbeianus (Shaw, 1802). Bull Environ Contam Toxicol 84:712–715. https://doi.org/10.1007/s00128-010-0014-0
Ossana NA, Castañé PM, Salibián A (2013) Use of Lithobates catesbeianus tadpoles in a multiple biomarker approach for the assessment of water quality of the Reconquista River (Argentina). Arch Environ Contam Toxicol 65:486–497. https://doi.org/10.1007/s00244-013-9920-6
Patterson S, Zee J, Wiseman S, Hecker M (2017) Effects of chronic exposure to dietary selenomethionine on the physiological stress response in juvenile white sturgeon (Acipenser transmontanus). Aquat Toxicol 186:77–86. https://doi.org/10.1016/j.aquatox.2017.02.003
Pinto Vidal FA, Abdalla FC, Carvalho C d S, Moraes Utsunomiya HS, Teixeira Oliveira LA, Salla RF, Jones-Costa M (2021a) Metamorphic acceleration following the exposure to lithium and selenium on American bullfrog tadpoles (Lithobates catesbeianus). Ecotoxicol Environ Saf 207:111101. https://doi.org/10.1016/j.ecoenv.2020.111101
Pinto Vidal FA, Carvalho C d S, Abdalla FC, Ceschi-Bertoli L, Moraes Utsunomiya HS, Henrique da Silva R, Salla RF, Jones-Costa M (2021b) Metabolic, immunologic, and histopathologic responses on premetamorphic American bullfrog (Lithobates catesbeianus) following exposure to lithium and selenium. Environ Pollut:116086. https://doi.org/10.1016/j.envpol.2020.116086
Regnault C, Worms IAM, Oger-Desfeux C, MelodeLima C, Veyrenc S, Bayle ML, Combourieu B, Bonin A, Renaud J, Raveton M, Reynaud S (2014) Impaired liver function in Xenopus tropicalis exposed to benzo[a]pyrene: transcriptomic and metabolic evidence. BMC Genomics 15:1–16. https://doi.org/10.1186/1471-2164-15-666
Salla RF, Gamero FU, Ribeiro LR, Rizzi GM, Dal Medico SE, Rissoli RZ, Vieira CA, Silvazacarin ECM, Leite DS, Abdalla FC, Toledo LF, Costa MJ (2015) Cardiac adaptations of bullfrog tadpoles in response to chytrid infection. J Exp Zool Part A Ecol Genet Physiol 323:487–496. https://doi.org/10.1002/jez.1945
Santos B, Ribeiro R, Domingues I, Pereira R, Soares AMVM, Lopes I (2013) Salinity and copper interactive effects on perez’s frog Pelophylax perezi. Environ Toxicol Chem 32:1864–1872. https://doi.org/10.1002/etc.2257
Sappington KG (2002) Development of aquatic life criteria for selenium: a regulatory perspective on critical issues and research needs. Aquat Toxicol 57:101–113. https://doi.org/10.1016/S0166-445X(01)00267-3
Snodgrass JW, Hopkins WA, Roe JH (2003) Relationships among developmental stage, metamorphic timing, and concentrations of elements in bullfrogs (Rana catesbeiana). Environ Toxicol Chem 22:1597e1604
Snodgrass JW, Hopkins WA, Broughton J, Gwinn D, Baionno JA, Burger J (2004) Species-specific responses of developing anurans to coal combustion wastes. Aquat Toxicol 66:171e182
Stadtman CT (1974) Selenium biochemistry. Science (80- ) 183:915–922. https://doi.org/10.1126/science.183.4128.915
Thomas JK, Wiseman S, Giesy JP, Janz DM (2013) Effects of chronic dietary selenomethionine exposure on repeat swimming performance, aerobic metabolism and methionine catabolism in adult zebrafish (Danio rerio). Aquat Toxicol 130-131:112–122. https://doi.org/10.1016/j.aquatox.2013.01.009
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30. https://doi.org/10.1046/j.1523-1739.2000.99084.x
U.S. EPA (2016) Aquatic life ambient water quality criterion for selenium - freshwater; Washington, D.C.
Vasseur P, Cossu-Leguille C (2003) Biomarkers and community indices as complementary tools for environmental safety. Environ Int 28:711–717. https://doi.org/10.1016/S0160-4120(02)00116-2
Wanger TC (2011) The lithium future-resources, recycling, and the environment. Conserv Lett 4:202–206. https://doi.org/10.1111/j.1755-263X.2011.00166.x
Welsh HH, Ollivier LM (1998) Stream amphibians as indicators of ecosystem stress:a case study from california’s redwoods. Ecol Appl 8(4):1118–1132. https://doi.org/10.1890/1051-0761(1998)008[1118:saaioe]2.0.co;2
Yologlu E, Ozmen M (2015) Low concentrations of metal mixture exposures have adverse effects on selected biomarkers of Xenopus laevis tadpoles. Aquat Toxicol 168:19–27. https://doi.org/10.1016/j.aquatox.2015.09.006
Yoshizato K, Nakajima Y (1980) Tissue degradation patterns of amphibian tadpole tail: histological and biochemical studies. Develop Growth Differ 22:579–588. https://doi.org/10.1111/j.1440-169X.1980.00579.x
Zhu W, Chang L, Zhao T, Wang B, Jiang J (2020) Remarkable metabolic reorganization and altered metabolic requirements in frog metamorphic climax. Front Zool 17:1–16. https://doi.org/10.1186/s12983-020-00378-6
Acknowledgements
The authors would like to acknowledge RECETOX research infrastructure (the Czech Ministry of Education, Youth and Sports: LM2018121) and the CETOCOEN EXCELLENCE Teaming 2 project supported by Horizon2020 (857560) and the Czech Ministry of Education, Youth and Sports (02.1.01/0.0/0.0/18_046/0015975).
Funding
CAPES (Coordination for the Improvement of Higher Level—or Education—Personnel) Scholarship for Social Demand Code 001 to FAPV. Brazilian Institute of Comparative Physiology INCT-FisC (CNPq 573921/2008-3 and FAPESP 2008/57712-4) grant to MJC and FAPESP (grant number 2017/03653-6) and the Brazilian National Council for Scientific and Technological Development CNPq (grant number 302812/2016-4) to FAPESP (no. 2011/50752-3).
Author information
Authors and Affiliations
Contributions
Felipe Augusto Pinto Vidal: Investigation, writing the original draft, data analysis, and methodology. Fábio Camargo Abdalla: Investigation, methodology, and writing—review and editing. Cleoni dos Santos Carvalho: writing—review and editing. Heidi Samantha Moraes Utsunomiya: Investigation. Raquel Fernanda Salla: Data curation and writing—review and editing. Monica Jones-Costa: Supervision, writing—review and editing—and project administration.
Corresponding author
Ethics declarations
Ethics approval
All the proceedings regarding the use of vertebrates were previously approved by the University’s Animal Ethics and Experimentation Committee (CEUA # 1397170117) from the Federal University of São Carlos, Brazil.
Consent to participate
Not applicable.
Consent of publication
All the authors agreed with the present publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinto-Vidal, .A., Carvalho, C.d.S., Abdalla, F.C. et al. Effects of lithium and selenium in the tail muscle of American bullfrog tadpoles (Lithobates catesbeianus) during premetamorphosis. Environ Sci Pollut Res 29, 1975–1984 (2022). https://doi.org/10.1007/s11356-021-15686-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15686-5