Abstract
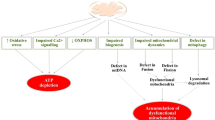
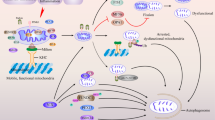
The mitochondria, apart from being known as the cell’s “powerhouse,” are crucial in the viability of nerve cells. Any damage to these cellular organelles can result in their cellular level dysfunction which includes rapidly multiplying reactive oxygen species (ROS) from the mitochondrial membrane, impaired calcium ion homeostasis, and disturbed mitochondrial dynamics by the formation of permeability transition pore in mitochondria. All these impaired biochemical changes lead to various neurological disorders such as progressive supranuclear palsy (PSP), Parkinson’s disease (PD), and Alzheimer’s disease (AD). Moreover, impaired mitochondrial functions are particularly prone to damage owing to prolonged lifespan and stretched length of the neurons. At the same time, neurons are highly dependent on ATP, and thus, the mitochondria play a central role in the pathogenesis pertaining to neuronal disorders. Dysfunction in the mitochondria is an early pathological hallmark of neurological disorders, and its early detection with the help of suitable biomarkers can lead to promising treatment in this area. Thus, the drugs which are targeting mitochondrial dysfunctions are the emerging area of research in connection with neurological disorders. This can be evidenced by the great opportunities for mitigation, diagnosis, and treatment of numerous human disorders that entail mitochondrial dysfunction at the nexus of their pathogenesis. Here, we throw light at the mitochondrial pathologies and indications of dysfunctional mitochondria in PD, AD, and PSP. There is also an insight into the possible therapeutic strategies highlighting the need for mitochondria-based medicine and made an attempt for claiming the prerequisite for the therapy of neurological diseases.





Similar content being viewed by others
Data availability
Not applicable.
References
Ahn D, Chung H, Lee HW, Kang K, Ko PW, Kim NS, Park T (2017) Smart gait-aid glasses for Parkinson’s disease patients. IEEE Trans Biomed Eng 64(10):2394–2402
Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 334(6059):1144–1147
Alam M, Schmidt WJ (2004) Mitochondrial complex I inhibition depletes plasma testosterone in the rotenone model of Parkinson’s disease. Physiol Behav 83(3):395–400
Albers DS, Beal MF (2002) Mitochondrial dysfunction in progressive supranuclear palsy. Neurochem Int 40(6):559–564
Albers DS, Augood SJ, Martin DM, Standaert DG, Vonsattel JP, Beal MF (1999) Evidence for oxidative stress in the subthalamic nucleus in progressive supranuclear palsy. J Neurochem 73(2):881–884
Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JP, Gibson GE, Beal MF (2000) Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment. J Neurochem 74(2):878–881
Alexeyev MF, LeDOUX SP, Wilson GL (2004) Mitochondrial DNA and aging. Clin Sci 107(4):355–364
Almeida S, Sarmento-Ribeiro AB, Januário C, Rego AC, Oliveira CR (2008) Evidence of apoptosis and mitochondrial abnormalities in peripheral blood cells of Huntington’s disease patients. Biochem Biophys Res Commun 374(4):599–603
Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA (2015) Mitochondrial ROS metabolism: 10 years later. Biochem Mosc 80(5):517–531
Aras S, Bai M, Lee I, Springett R, Hüttemann M, Grossman LI (2015) MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion. 20:43–51
Arenas J, Campos Y, Ribacoba R, Martín MA, Rubio JC, Ablanedo P, Cabello A (1998) Complex I defect in muscle from patients with Huntington’s disease. Ann Neurol 43(3):397–400
Ascherio A, Weisskopf MG, O'Reilly EJ, Jacobs EJ, McCullough ML, Calle EE, Cudkowicz M, Thun MJ (2005) Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol 57(1):104–110
Bacman SR, Williams SL, Hernandez D, Moraes CT (2007) Modulating mtDNA heteroplasmy by mitochondria-targeted restriction endonucleases in a ‘differential multiple cleavage-site’ model. Gene Ther 14(18):1309–1318
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 434(7033):658–662
Baloyannis SJ, Costa V, Michmizos D et al (2004) Am J Alzheimers Dis Other Dement 19(2):89–93
Banerjee R, Starkov AA, Beal MF, Thomas B (2009) Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta (BBA)-Mol Basis Dis 1792(7):651–663
Barbiroli B, Iotti S, Cortelli P, Martinelli P, Lodi R, Carelli V, Montagna P (1999) Low brain intracellular free magnesium in mitochondrial cytopathies. J Cereb Blood Flow Metab 19:528–532
Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT (2005) Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci 102(40):14392–14397
Beal MF (2003) Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol 53(S3):S39–S48
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58(4):495–505
Beal MF, Matthews RT, Tieleman A, Shults CW (1998) Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res 783(1):109–114
Becker T, Gebert M, Pfanner N, van der Laan M (2009) Biogenesis of mitochondrial membrane proteins. Curr Opin Cell Biol 21(4):484–493
Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120:838–848
Bi F, Li F, Huang C, Zhou H (2013) Pathogenic mutation in VPS35 impairs its protection against MPP+ cytotoxicity. Int J Biol Sci 9(2):149–155
Bohr VA (2002) Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med 32(9):804–812
Bonda DJ, Wang X, Lee HG, Smith MA, Perry G, Zhu X (2014) Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull 30(2):243–252
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Phys Cell Phys 287(4):C817–C833
Cabiscol E, Piulats E, Echave P, Herrero E, Ros J (2000) Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem 275(35):27393–27398
Cardoso SM, Proença MT, Santos S, Santana I, Oliveira CR (2004a) Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging 25(1):105–110
Cardoso SM, Santana I, Swerdlow RH, Oliveira CR (2004b) Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J Neurochem 89(6):1417–1426
Cardoso S, Carvalho C, Correia SC, Seiça RM, Moreira PI (2016) Alzheimer’s disease: from mitochondrial perturbations to mitochondrial medicine. Brain Pathol 26(5):632–647
Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Du Yan S (2005) Mitochondrial Aβ: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19(14):2040–2041
Cavallini G, Donati A, Taddei M, Bergamini E (2007) Evidence for selective mitochondrial autophagy and failure in aging. Autophagy. 3(1):26–27
Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Praticò D (2004) Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem 90(3):758–764
Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci 101(29):10726–10731
Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA (2010) The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biomed Sci Med Sci 65(2):119–128
Crompton M, Barksby E, Johnson N, Capano M (2002) Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 84(2-3):143–152
Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G (2006) Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem 96(5):1349–1361
Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26(35):9057–9068
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23(4):394–400
Ding WX, Yin XM (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393(7):547–564
Du H, Yan SS (2010) Mitochondrial medicine for neurodegenerative diseases. Int J Biochem Cell Biol 42(5):560–572
Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14(10):1097–1105
Dupuis L, De Aguilar JL, Oudart H, De Tapia M, Barbeito L, Loeffler JP (2004) Mitochondria in amyotrophic lateral sclerosis: a trigger and a target. Neurodegener Dis 1(6):245–254
Echaniz-Laguna A, Zoll J, Ponsot E, N'guessan B, Tranchant C, Loeffler JP, Lampert E (2006) Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp Neurol 198(1):25–30
Edgar D, Trifunovic A (2009) The mtDNA mutator mouse: dissecting mitochondrial involvement in aging. Aging (Albany NY) 1(12):1028
Etminan M, Gill SS, Samii A (2005) Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol 4(6):362–365
Flint BM (2002) Coenzyme Q 10 as a possible treatment for neurodegenerative diseases. Free Radic Res 36(4):455–460
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66(2):137–147
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1(4):515–525
Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N (2004) Ca2+ homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem 279(21):22704–22714
Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S, Li Y, Ogaki K, Ando M, Yoshino H, Tomiyama H (2015) CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol 14(3):274–282
Gaugler J, James B, Johnson T, Marin A, Weuve J (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15(3):321–387
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12(2):119–131
Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF (2010) Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta (BBA)-Mol Basis Dis 1802(1):122–134
Gordeeva AV, Zvyagilskaya RA, Labas YA (2003) Cross-talk between reactive oxygen species and calcium in living cells. Biochem Mosc 68(10):1077–1080
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science. 305(5684):626–629
Grivennikova VG, Vinogradov AD (2006) Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta (BBA)-Bioenergetics 1757(5-6):553–561
Gu G, Reyes PF, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J (2002) Mitochondrial DNA deletions/rearrangements in Parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol 61(7):634–639
Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA (2005) Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat 29(3):173–178
Gutzmann H, Hadler D (1998) Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a 2-year double-blind multicentre study. J Neural Transm Suppl 54:301–310
Guzmán DC, Jiménez FT, García EH, Olguín HJ (2007) Assessment of antioxidant effect of 2, 5-dihydroxybenzoic acid and vitamin A in brains of rats with induced hyperoxia. Neurochem Res 32(6):1036–1040
Halestrap AP (2006) Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 34(Pt 2):232–237
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61(3):372–385
Hanagasi HA, Ayribas D, Baysal K, Emre M (2005) Mitochondrial complex I, II/III, and IV activities in familial and sporadic Parkinson’s disease. Int J Neurosci 115(4):479–493
Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM (1995) Direct evidence of oxidative injury produced by the Alzheimer’s β-amyloid peptide (1–40) in cultured hippocampal neurons. Exp Neurol 131(2):193–202
Harrison FE, Hosseini AH, McDonald MP, May JM (2009) Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav 93(4):443–450
Henry W, Querfurth HW, LaFerla FM (2010) Mechanisms of disease Alzheimer’s disease. New Engl J Med 362:329–344
Hervias I, Beal MF, Manfredi G (2006) Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve 33(5):598–608
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21(9):3017–3023
Hong WK, Han EH, Kim DG, Ahn JY, Park JS, Han BG (2007) Amyloid-β-peptide reduces the expression level of mitochondrial cytochrome oxidase subunits. Neurochem Res 32(9):1483–1488
Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, Elsworth JD, Sotonyi P, Roth RH, Dietrich EH, Matthews RT (2003) Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology. 144(7):2757–2760
Hsieh CH, Shaltouki A, Gonzalez AE, da Cruz AB, Burbulla LF, Lawrence ES, Schüle B, Krainc D, Palmer TD, Wang X (2016) Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell 19(6):709–724
Ishige K, Schubert D, Sagara Y (2001) Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 30(4):433–446
James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, Murphy MP (2007) Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem 282(20):14708–14718
Jauslin ML, Meier T, Smith RA, Murphy PM (2003) Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17(13):1–10
Jenner P (1996) Oxidative stress in Parkinson’s disease and other neurodegenerative disorders. Pathologie-biologie. 44(1):57–64
Jeong SY, Seol DW (2008) The role of mitochondria in apoptosis. BMB Rep 41(1):11–22
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392(6676):605–608
Koene S, Smeitink J (2011) Mitochondrial medicine. J Inherit Metab Dis 34(2):247–248
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 309(5733):481–484
Kurzer MS, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17(1):353–381
Lee JE, Westrate LM, Wu H, Page C, Voeltz GK (2016) Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 540(7631):139–143
Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE (2005) Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Phys Regul Integr Comp Phys 288(5):R1288–R1296
Leung AW, Halestrap AP (2008) Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta (BBA)-Bioenergetics 1777(7-8):946–952
Lill CM (2016) Genetics of Parkinson’s disease. Mol Cell Probes 30(6):386–396
Littarru GP, Tiano L (2007) Bioenergetic and antioxidant properties of coenzyme Q 10: recent developments. Mol Biotechnol 37(1):31–37
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55(1):97–105
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80(5):780–787
Logroscino G (2005) The role of early-life environmental risk factors in Parkinson disease: what is the evidence? Environ Health Perspect 113:1234–1238
Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R (1996) Dietary lipids and antioxidants in Parkinson’s disease: a population-based, case-control study. Ann Neurol 39(1):89–94
Luchsinger JA, Tang MX, Shea S, Mayeux R (2003) Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol 60(2):203–208
Luft R (1994) The development of mitochondrial medicine. Proc Natl Acad Sci 91(19):8731–8738
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F (2004) ABAD directly links Aß to mitochondrial toxicity in Alzheimer’s disease. Science. 304(5669):448–452
Martinelli P, Scaglione C, Lodi R, Iotti S, Barbiroli B (2000) Deficit of brain and skeletal muscle bioenergetics in progressive supranuclear palsy shown in vivo by phosphorus magnetic resonance spectroscopy. Mov Disord 15(5):889–893
Martínez-Rumayor A, Arrieta O, Sotelo J, García E (2009) Female gender but not cigarette smoking delays the onset of Parkinson’s disease. Clin Neurol Neurosurg 111(9):738–741
Martinou JC, Green DR (2001) Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2(1):63–67
Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41(12):1234–1238
Masiero E, Sandri M (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy. 6(2):307–309
Maurer I, Zierz S, Möller HJ (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging 21(3):455–462
McGirr S, Venegas C, Swaminathan A (2020) Alzheimer’s disease: a brief review. J Exp Neurol 29:1(3)
McIntosh LJ, Trush MA, Troncoso JC (1997) Increased susceptibility of Alzheimer’s disease temporal cortex to oxygen free radical-mediated processes. Free Radic Biol Med 23(2):183–190
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Vbeyreuther K, Bush AI, Masters CL (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46(6):860–866
Miller IN, Cronin-Golomb A (2010) Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord 25(16):2695–2703
Miller ER III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142(1):37–46
Möller HJ, Graeber MB (1998) The case described by Alois Alzheimer in 1911. Eur Arch Psychiatry Clin Neurosci 248(3):111–122
Monte DA, Harati Y, Jankovic J, Sandy MS, Jewell SA, Langston JW (1994) Rapid communication muscle mitochondrial ATP production in progressive supranuclear palsy. J Neurochem 62(4):1631–1634
Muller FL, Liu Y, Van Remmen H (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279(47):49064–49073
Murata T, Ohtsuka C, Terayama Y (2008) Increased mitochondrial oxidative damage and oxidative DNA damage contributes to the neurodegenerative process in sporadic amyotrophic lateral sclerosis. Free Radic Res 42(3):221–225
Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, Antonsson B, Pandey S (2006) Water-soluble formulation of Coenzyme Q 10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis. 11(8):1359–1369
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278(10):7743–7746
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH (2010) Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta (BBA)-Mol Basis Dis 1802(1):228–234
Osiewacz HD, Bernhardt D (2013) Mitochondrial quality control: impact on aging and life span-a mini-review. Gerontology. 59(5):413–420
Palmer CS, Osellame LD, Stojanovski D, Ryan MT (2011) The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 23(10):1534–1545
Panickar KS, Polansky MM, Anderson RA (2009) Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen-glucose deprivation in cultures. Nutr Neurosci 12(3):105–113
Panov AV, Lund S, Greenamyre JT (2005) Ca 2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem 269(1):143–152
Parikh S, Saneto R, Falk MJ, Anselm I, Cohen BH, Haas R (2009) A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol 11(6):414–430
Park S, Kim H, Lee J, Yoon K, Chang M, Park S (2010) The age-dependent induction of apoptosis-inducing factor (AIF) in the human semitendinosus skeletal muscle. Cell Mol Biol Lett 15(1):1–2
Park JS, Davis RL, Sue CM (2018) Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 18(5):21
Parkinson JA (1817) An essay on the shaking palsy (London: Sherwood, Neely and Jones). J Neuropsychiatr Clin Neurosci 14(2):223–236
Perez Ortiz JM, Swerdlow RH (2019) Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176(18):3489–3507
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 300(5622):1140–1142
Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF (2006) Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98(4):1141–1148
Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 85(2):257–273
Quintanilla RA, Johnson GV (2009) Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res Bull 80(4-5):242–247
Reddy PH (2008) Mitochondrial medicine for aging and neurodegenerative diseases. NeuroMolecular Med 10(4):291–315
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14(2):45–53
Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J (2005) Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25(38):8807–8814
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E (2009) Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci 106(47):20057–20062
Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA (2010) Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 48(1):161–172
Rosenstock TR, Carvalho AC, Jurkiewicz A, Frussa-Filho R, Smaili SS (2004) Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J Neurochem 88(5):1220–1228
Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B (2013) Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 155(6):1351–1364
Ryan BJ, Hoek S, Fon EA, Wade-Martins R (2015) Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci 40(4):200–210
Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114(5):867–874
Sanz A, Caro P, Gómez J, Barja G (2006) Testing the vicious cycle theory of mitochondrial ROS production: effects of H 2 O 2 and cumene hydroperoxide treatment on heart mitochondria. J Bioenerg Biomembr 38(2):121–127
Sato M, Sato K (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 334(6059):1141–1144
Scarffe LA, Stevens DA, Dawson VL, Dawson TM (2014) Parkin and PINK1: much more than mitophagy. Trends Neurosci 37(6):315–324
Selkoe DJ (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140(8):627–638
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci 102(15):5618–5623
Smirnova E, Griparic L, Shurland DL, Van Der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12(8):2245–2256
Smith RA, Porteous CM, Coulter CV, Murphy MP (1999) Selective targeting of an antioxidant to mitochondria. Eur J Biochem 263(3):709–716
Stuart JA, Hashiguchi K, Wilson DM III, Copeland WC, Souza-Pinto NC, Bohr VA (2004) DNA base excision repair activities and pathway function in mitochondrial and cellular lysates from cells lacking mitochondrial DNA. Nucleic Acids Res 32(7):2181–2192
Sugioka R, Shimizu S, Tsujimoto Y (2004) Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279(50):52726–52734
Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Praticò D (2004) Early vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J 18(2):323–325
Surh YJ, Lee JY, Choi KJ, Ko SR (2002) Effects of selected ginsenosides on phorbol ester-induced expression of cyclooxygenase-2 and activation of NF-κB and ERK1/2 in mouse skin. Ann N Y Acad Sci 973(1):396–401
Swerdlow RH (2002) Mitochondrial DNA–related mitochondrial dysfunction in neurodegenerative diseases. Arch Pathol Lab Med 126(3):271–280
Swerdlow RH (2009) Mitochondrial medicine and the neurodegenerative mitochondriopathies. Pharmaceuticals. 2(3):150–167
Swerdlow RH, Khan SM (2004) A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses 63(1):8–20
Swerdlow RH, Khan SM (2009) The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol 218(2):308–315
Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta (BBA)-Mol Basis Dis 1842(8):1219–1231
Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16(1):59–68
Szeto HH (2006) Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8(2):E277–E283
Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS (2005) ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J 19(6):1–25
Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T (2009) RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proc Natl Acad Sci 106(47):20021–20026
Tan L (2013) Epidemiology of Parkinson’s disease. Neurol Asia 18(3)
Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC (2015) VPS35 deficiency or mutation causes dopaminergic neuronal loss by impairing mitochondrial fusion and function. Cell Rep 12(10):1631–1643
Toledo JB, Arnold M, Kastenmüller G, Chang R, Baillie RA, Han X, Thambisetty M, Tenenbaum JD, Suhre K, Thompson JW, John-Williams LS (2017) Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement 13(9):965–984
Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG (2005) Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci 102(50):17993–17998
Trimmer PA, Keeney PM, Borland MK, Simon FA, Almeida J, Swerdlow RH, Parks JP, Parker WD Jr, Bennett JP Jr (2004) Mitochondrial abnormalities in cybrid cell models of sporadic Alzheimer’s disease worsen with passage in culture. Neurobiol Dis 15(1):29–39
Twelves D, Perkins KS, Counsell C (2003) Systematic review of incidence studies of Parkinson’s disease. Mov Disord 18(1):19–31
Wallace DC (2010) Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen 51(5):440–450
Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA (2005) Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem 93(4):953–962
Wang X, Su BO, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta (BBA)-Mol Basis Dis 1842(8):1240–1247
Weissig V (2003) Mitochondrial-targeted drug and DNA delivery. Crit Rev Ther Drug Carrier Syst 20(1)
Weissig V, Edeas M (eds) (2015) Mitochondrial medicine. Humana Press, Totowa
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci 106(48):20405–20410
Winkler-Stuck K, Kirches E, Mawrin C, Dietzmann K, Lins H, Wallesch CW, Kunz WS, Wiedemann FR (2005) Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson’s disease. J Neural Transm 112(4):499–518
Yang W, Hekimi S (2010) A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8(12):e1000556
Yang X, Yang Y, Li G, Wang J, Yang ES (2008a) Coenzyme Q10 attenuates β-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci 34(2):165–171
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008b) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci 105(19):7070–7075
Young AJ, Johnson S, Steffens DC, Doraiswamy PM (2007) Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr 12(1):62–68
Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103:2653–2658
Yu JT, Chang RC, Tan L (2009) Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol 89(3):240–255
Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC (2004) Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol 61(1):82–88
Zhang SM, Hernan MA, Chen H, Spiegelman D, Willett WC, Ascherio A (2002) Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 59(8):1161–1169
Zheng Z, Zhao H, Steinberg GK, Yenari MA (2003) Cellular and molecular events underlying ischemia-induced neuronal apoptosis. Drug News Perspect 16(8):497–503
Zhou C, Huang Y, Przedborski S (2008) Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci 1147:93–104
Zhou L, Wang W, Hoppel C, Liu J, Zhu X (2017) Parkinson’s disease-associated pathogenic VPS35 mutation causes complex I deficits. Biochim Biophys Acta (BBA)-Mol Basis Dis 1863(11):2791–2795
Acknowledgements
The authors would like to thank the Chitkara University, Punjab, India, for providing the basic facilities for completion of the current article.
Author information
Authors and Affiliations
Contributions
A.A. and T.B.: conceived the idea and wrote the first draft. A.S.: figure work. S.S. and N.S.: data compilation. S.B.: proof read
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arora, A., Behl, T., Sehgal, A. et al. Targeting cellular batteries for the therapy of neurological diseases. Environ Sci Pollut Res 28, 41517–41532 (2021). https://doi.org/10.1007/s11356-021-14665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14665-0