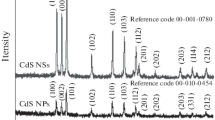
Abstract
Capping agent-free CdS quantum dots (CdS-QDs) were synthesized within the mesopores of MCM-41 and interlayers of montmorillonite (MMT), using a safe manner by a facile ion exchange-precipitation protocol. The mesopores of MCM-41 and interlayers of MMT controlled the growth of CdS-QDs. The obtained CdS-QDs@MCM-41 and CdS-QDs/MMT were characterized by X-ray diffraction (XRD) analysis, energy-dispersive X-ray (EDX), diffuse reflectance UV-Vis, and photoluminescence spectroscopies. Photodegradation of rhodamine-B (RhB) over these embedded CdS-QDs was investigated under UV-Vis light irradiation. The influences of some parameters on the photodegradation of RhB such as pH, temperature, and UV-Vis irradiation time were investigated. The results showed that the CdS-QDs/MMT and CdS-QDs@MCM-41 have high efficiencies for RhB photodegradation under UV-Vis illumination.









Similar content being viewed by others
References
Aboulaich A, Billaud D, Abyan M et al (2012) One-pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl Mater Interfaces 4(5):2561–2569
Cai Q, Lin W-Y, Xiao F-S et al (1999) The preparation of highly ordered MCM-41 with extremely low surfactant concentration. Microporous Mesoporous Mater 32(1):1–15
Chen X, Wu Z, Liu D et al (2017) Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res Lett 12(1):143
Cheng H, Wang Y, Dai H et al (2015) Nonlinear optical properties of PbS colloidal quantum dots fabricated via solvothermal method. J Phys Chem C 119(6):3288–3292
Empedocles S, Bawendi M (1999) Spectroscopy of single CdSe nanocrystallites. Acc Chem Res 32(5):389–396
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37
Gao Y, Peng X (2014) Crystal structure control of CdSe nanocrystals in growth and nucleation: dominating effects of surface versus interior structure. J Am Chem Soc 136(18):6724–6732
Ghanbari M, Salavati-Niasari M (2018) Tl4CdI6 Nanostructures: facile sonochemical synthesis and photocatalytic activity for removal of organic dyes. Inorg Chem 57:11443–11455
Haro M, Abargues R, Herraiz-Cardona I et al (2014) Plasmonic versus catalytic effect of gold nanoparticles on mesoporous TiO2 electrodes for water splitting. Electrochim Acta 144:64–70
Hung W-H, Chien T-M, Tseng C-M (2014) Enhanced photocatalytic water splitting by plasmonic TiO2–Fe2O3 cocatalyst under visible light irradiation. J Phys Chem C 118(24):12676–12681
Kaur R, Vellingiri K, Kim K-H et al (2016) Efficient photocatalytic degradation of rhodamine 6G with a quantum dot-metal organic framework nanocomposite. Chemosphere 154:620–627
Khaorapapong N, Ontam A, Youngme S et al (2008) Solid-state intercalation and in situ formation of cadmium sulfide in the interlayer space of montmorillonite. J Phys Chem Solids 69(5-6):1107–1111
Kim HN, Kim TW, Kim IY et al (2011) Cocatalyst-free photocatalysts for efficient visible-light-induced H2 production: porous assemblies of CdS quantum dots and layered titanate nanosheets. Adv Funct Mater 21(16):3111–3118
Li G-S, Zhang D-Q, Yu JC (2009) A new visible-light photocatalyst: CdS quantum dots embedded mesoporous TiO2. Environ Sci Technol 43(18):7079–7085
Lim J, Park Y-S, Klimov VI (2018) Optical gain in colloidal quantum dots achieved with direct-current electrical pumping. Nat Mater 17(1):42
Mandal P, Talwar S, Major S et al (2008) Orange-red luminescence from Cu doped CdS nanophosphor prepared using mixed Langmuir–Blodgett multilayers. J Chem Phys 128(11):114703
Mehdizadeh P, Orooji Y et al (2020) Green synthesis using cherry and orange juice and characterization of TbFeO3 ceramic nanostructures and their application as photocatalysts under UV light for removal of organic dyes in water. J Clean Prod 252:119765
Naseri A, Samadi M, Pourjavadi A et al (2017) Graphitic carbon nitride (gC3N4)-based photocatalysts for solar hydrogen generation: recent advances and future development directions. J Mater Chem A Mater 5(45):23406–23433
Qin Q, Ma J, Liu K (2009) Adsorption of anionic dyes on ammonium-functionalized MCM-41. J Hazard Mater 162(1):133–139
Qu Q, Liu Y, Tang X et al (2006) Etched bare fused-silica capillaries for online preconcentration of amino acids in CE. Electrophoresis 27(22):4500–4507
Rajamanickam D, Shanthi M (2016) Photocatalytic degradation of an organic pollutant by zinc oxide–solar process. Arab J Chem 9:S1858–S1868
Saison T, Chemin N, Chanéac C et al (2015) New insights into BiVO4 properties as visible light photocatalyst. J Phys Chem C 119(23):12967–12977
Samadi-Maybodi A, Sadeghi-Maleki MR (2016) In-situ synthesis of high stable CdS quantum dots and their application for photocatalytic degradation of dyes. Spectrochim Acta A Mol Biomol Spectrosc 152:156–164
Shamsipur M, Rajabi HR (2014) Study of photocatalytic activity of ZnS quantum dots as efficient nanoparticles for removal of methyl violet: effect of ferric ion doping. Spectrochim Acta A Mol Biomol Spectrosc 122:260–267
Shao Y, Wang X, Kang Y et al (2014) Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J Colloid Interface Sci 429:25–33
Shen S, Guo L (2008) Growth of quantum-confined CdS nanoparticles inside Ti-MCM-41 as a visible light photocatalyst. Mater Res Bull 43(2):437–446
Sun Y, Liu Q, Gao S et al (2013) Pits confined in ultrathin cerium (IV) oxide for studying catalytic centers in carbon monoxide oxidation. Nat Commun 4:2899
Tian J, Leng Y, Zhao Z et al (2015) Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 11:419–427
Valenti M, Kontoleta E, Digdaya IA et al (2016) The role of size and dimerization of decorating plasmonic silver nanoparticles on the photoelectrochemical solar water splitting performance of BiVO4 photoanodes. ChemNanoMat 2(7):739–747
Wang P, Zhu Y, Yang X et al (2007) Electrochemical synthesis of magnetic nanoparticles within mesoporous silica microspheres. Colloids Surf A Physicochem Eng Asp 294(1):287–291
Wang J, Lim Y-F, Ho GW (2014) Carbon-ensemble-manipulated ZnS heterostructures for enhanced photocatalytic H2 evolution. Nanoscale 6(16):9673–9680
Wang J, Xu W, Bao H et al (2015) Synthesis of ordered mesoporous crystalline CuS and Ag2S materials via cation exchange reaction. Nanoscale 7(10):4468–4474
Weller H, Schmidt H, Koch U et al (1986) Photochemistry of colloidal semiconductors. Onset of light absorption as a function of size of extremely small CdS particles. Chem Phys Lett 124(6):557–560
Xue J, Mao W, Wang Y et al (2011) Preparation of spherical chitosan resin and adsorption of methylene blue. Rare Metals 30(1):249–253
Zhao Y, Chen S, Sun B et al (2015) Graphene-Co3O4 nanocomposite as electrocatalyst with high performance for oxygen evolution reaction. Sci Rep 5:7629
Zhu L, Meng Z-D, Park C-Y et al (2013) Characterization and relative sonocatalytic efficiencies of a new MWCNT and CdS modified TiO2 catalysts and their application in the sonocatalytic degradation of rhodamine B. Ultrason Sonochem 20(1):478–484
Zinatloo-Ajabshir S, Morassaei MS et al (2020) Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram Int 46:6095–6107
Funding
The authors received financial support (Grant Number H/4/361) from Kharazmi University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Masteri-Farahani, M., Mosleh, N. CdS quantum dots encapsulated within the mesopores of MCM-41 and interlayers of montmorillonite as photocatalysts for rhodamine-B degradation in aqueous solution. Environ Sci Pollut Res 28, 4615–4622 (2021). https://doi.org/10.1007/s11356-020-10810-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10810-3