Abstract
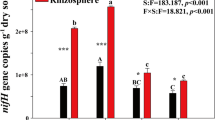
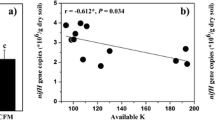
Diazotrophy is considered as one of the most crucial and dynamic phenomena in the rice field and also a major source of nitrogen input. The objective of this study was to elucidate possible interactions between diverse and dominant diazotrophic bacterial community and organic carbon composition of the paddy soil. Our results suggest that most abundantly found diazotrophs belong to a proteobacteria group and uncultured bacterial forms. A gene abundance study clearly showed significantly higher diazotrophic abundance (P < 0.01) at Chandauli (CHN) as compared to Varanasi (VNS) and Ghazipur (GHJ) districts of Eastern Uttar Pradesh, India, with nitrogenase reductase (nifH) copy number between 1.44 × 103 and 3.34 × 103 copy g−1 soil. Fourier-transform infrared (FT-IR) spectroscopy data identified –CO–, C=O (\( {\mathrm{NH}}_{2^{-}} \) and –NH–), \( {\mathrm{CH}}_{2^{-}} \), and OH– as dominant organic functional groups in the paddy soil. Multivariate analysis was performed to get a clear and more accurate picture of interactions between free-living diazotrophs and abiotic soil factors. Regression analysis suggested a similar trend of distribution of different functional groups along each site. Relative abundance and diversity of diazotrophic population increased in response to FT-IR-based soil organic fractions. Maximum number of FT-IR spectral peak at sites in the Chandauli district augmented its bacterial diazotrophic diversity and abundance. Taken together, the present study sheds light on the substrate-driven composition of the microbial population of selected paddy areas.









Similar content being viewed by others
References
Adt I, Kohler A, Gognies S, Budin J, Sandt C, Belarbi A, Manfait M, Sockalingum GD (2010) FTIR spectroscopic discrimination of Saccharomyces cerevisiae and Saccharomyces bayanus strains. Can J Microbiol 56:793–801
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Phylogenet Evol 16:37–48
Bandick AK, Dick PR (1999) Field management effects on soil enzyme activities. Soil Biol Biochem 31:1471–1479
Banerjee A, Supakar S, Banerjee R (2014) Melanin from the nitrogen-fixing bacterium Azotobacter chroococcum: a spectroscopic characterization. PLoS One 9:e84574. https://doi.org/10.1371/journal.pone.0084574
Barberán A, Bates ST, Casamayor EO, Fierer N (2012) Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J 6:343–351
Bardgett RD, Mawdsley JL, Edwards S, Hobbs PJ, Rodwell JS, Davis WJ (1999) Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Funct Ecol 13:650–660
Bouskill NJ, Wood TE, Baran R, Hao Z, Ye Z, Bowen BP, Lim H, Nico PS, Holman HY, Gilbert B, Silver WL, Northen TR, Brodie EL (2016) Belowground response to drought in a tropical forest soil II change in microbial function impacts carbon composition. Front Microbiol 7:323. https://doi.org/10.3389/fmicb.2016.00323
Cancela GD, Taboada ER, Huertas FJ, Laguna AH, Rasero FS (1996) Interaction of trialkyl phosphites with montmorillonites. Clay Clay Miner 44:170–176
Cassens I, Mardulyn P, Milinkovitch MC (2005) Evaluating intraspecific “network” construction methods using simulated sequence data do existing algorithms outperform the global maximum parsimony approach. Syst Biol 54:363–372
Chaffron S, Rehrauer H, Pernthaler J, Von Mering C (2010) A global network of coexisting microbes from environmental and whole-genome sequence data. Genome Res 20:947–959. https://doi.org/10.1101/gr.104521.109
Chen YB, Dominic B, Mellon MT, Zehr JP (1998) Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. Strain IMS 101. J Bacteriol 180:3598–3605
Chen J, ZT Y, Michel FC, Wittum T, Morrison M (2007) Development and application of real-time PCR assays for quantification of erm genes conferring resistance to macrolides-lincosamides-streptogramin B in livestock manure and manure management systems. Appl Environ Microbiol 73:4407–4416
Cho J-C, Giovannoni SJ (2004) Cultivation and growth characteristics of a diverse group of oligotrophic marine Gammaproteobacteria. Appl Environ Microbiol 70:432–440
Choo-Smith LP et al (2001) Investigating microbiol (micro)colony heterogeneity by vibrational spectroscopy. Appl Environ Microbiol 67:1461–1469
Chow CET, Sachdeva R, Cram JA, Steele JA, Needham DM, Patel A et al (2013) Temporal variability and coherence of euphotic zone bacterial communities over a decade in the Southern California Bight. ISME J 7:2259–2273
Farnelid H, Andersson AF, Bertilsson S, Al-Soud WA, Hansen LH, Sørensen S, Steward GF, Hagström Å, Riemann L (2011) Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PLoS One 6:e19223
Farnelid H, Bentzon-Tilia M, Andersson AF, Bertilsson S, Jost G, Labrenz M, Jürgens K, Riemann L (2013) Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J 7(7):1413–1423
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Grangeteau C, Gerhards D, Rousseaux S, von Wallbrunn C, Alexandre H, Guilloux-Benatier M (2015) Diversity of yeast strains of the genus Hanseniaspora in the winery environment: what is their involvement in grape must fermentation? Food Microbiol 50:70–77
Grube M, Muter O, Strikauska S, Gavare M, Limane B (2008) Application of FT-IR spectroscopy for control of the medium composition during the biodegradation of nitro aromatic compounds. J Ind Microbiol Biotechnol 35:1545–1549
Hammer O, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hayden HL, Drake J, Imhof M, Oxley APA, Norng S, Mele PM (2010) The abundance of nitrogen cycle genes amoA and nifH depends on land-uses and soil types in South-Eastern Australia. Soil Biol Biochem 42:1774–1783
Hoover T (2000) Control of nitrogen fixation genes in Klebsiella pneumoniae. In: Triplett EW (ed) Prokaryotic nitrogen fixation. Horizon Scientific Press, Norfolk England, pp 131–147
Hsu SF, Buckley DH (2009) Evidence for the functional significance of diazotroph community structure in soil. ISME J 3:124–136
Ikenaga M, Muraoka Y, Toyota K, Kimura M (2002) Community structure of the microbiota associated with nodal roots of rice plants along with the growth stages: estimation by PCR-RFLP analysis. Biol Fertil Soils 36:397–404
Kennedy AC, Smith KL (1995) Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 170:75–86
Kim K, Zhang YP, Roberts GP (1999) Correlation of activity regulation and substrate recognition of the ADP ribosyltransferase that regulates nitrogenase activity in Rhodospirillum rubrum. J Bacteriol 181:1698–1702
Knief C, Delmotte N, Chaffron S, Stark M, Innerebner G, Wassmann R, von Mering C, Vorholt JA (2012) Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J 6:1378–1390
Koberl M, Erlacher A, Ramadan E et al (2016) Comparisons of diazotrophic communities in native and agricultural desert ecosystems reveal plants as important drivers in diversity. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiv166
Kögel-Knabner I (2000) Analytical approaches for characterizing soil organic matter. Org Geochem 31:609–625
Ladha JK, Reddy PM (2003) Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252(1):151–167
Limmer C, Drake HL (1998) Effect of carbon nitrogen and electron acceptor availablity on anaerobic N2 fixation in beech forest soil. Soil Biol Biochem 30:153–158
Liu JY, Peng MJ, Li YG (2012) Phylogenetic diversity of nitrogen-fixing bacteria and the nifH gene from mangrove rhizosphere soil. Can J Microbiol 58:531–539
Lopes AR, Bello D, Prieto-Fernández Á, Trasar-Cepeda C, Manaia CM, Nunes OC (2015) Relationships among bulk soil physicochemical biochemical and microbiological parameters in an organic alfalfa-rice rotation system. Environ Science Pollution Res 22:11690–11699
Miguel MAG, Bratos MAP, Martin FJG, Dueňas AD, Martin JFR, Gutiĕrrez PR, Orduňa AD, Rodrǐguez AT (2003) Identification of species of Brucella using Fourier transform infrared spectroscopy. J Microbiol Methods 55:121–131
Naumann D, Helm D, Labischinski H (1991) Microbiological characterizations by FT-IR spectroscopy. Nature 351:81–82
Nichols P, Henson M, Guckert J, Nivens J, White DC (1985) Fourier transform-infrared spectroscopic methods for microbial ecology: analysis of bacteria bacteria-polymer mixtures and biofilms. J Microbial Methods 4:79–94
Padmanabhan P, Padmanabhan S, DeRito C, Gray A, Gannon D, Snape JR, Tsai CS, Park W, Jeon C, Madsen EL (2003) Respiration of 13C-labeled substrates added to soil in the field and subsequent 16S rRNA gene analysis of 13C-labeled soil DNA. Appl Environ Microbiol 69:1614–1622
Poly F, Ranjard L, Nazaret S, Gourbière F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Riemann L, Farnelid H, Steward GF (2010) Nitrogenase genes in non-cyanobacterial plankton: prevalence diversity and regulation in marine waters. Aquat Microb Ecol 61:235–247
Riffkin PA, Quigley PE, Kearney GA, Cameron FJ, Gault RR, Peoples MB, Thies JE (1999) Factors associated with biological nitrogen fixation in dairy pastures in south-western Victoria. Aust J Agric Res 50:261–272
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shu W, Pablo GP, Jun Y, Danfeng H (2012) Abundance and diversity of nitrogen-fixing bacteria in rhizosphere and bulk paddy soil under different duration of organic management. World J Microbiol Biotechnol 28:493–503
Snajdr J, Cajthaml T, Val askov a V, Merhautova V, Petr ankov a M, Spetz P, Leppänen K, Baldrian P (2011) Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75:291–303
Srivastava M, Kaushik MS, Mishra AK (2016a) Linking the physico-chemical properties with the abundance and diversity of rhizospheric bacterial population inhabiting paddy soil based on a concerted multivariate analysis of PCR-DGGE and ribosomal intergenic spacer analysis (RISA). Geomicrobiol J 33:894–905
Srivastava M, Kaushik MS, Singh A, Singh D, Mishra AK (2016b) Molecular phylogeny of heterotrophic nitrifiers and aerobic denitrifiers and their potential role in ammonium removal. J Basic Microbiol 56:907–921
Srivastava M, Kaushik MS, Srivastava A, Singh A, Verma E, Mishra AK (2016c) Deciphering the evolutionary affiliations among bacterial strains (Pseudomonas and Frankia sp) inhabiting same ecological niche using virtual RFLP and simulation-based approaches. 3 Biotech 6:178
Udelhoven T, Naumann D, Schmit J (2000) Development of a hierarchical classification system with artificial neural networks and FTIR spectra for the identification of bacteria. Appl Spectrosc 54(10):1471–1479
Verhoefen MK, Schäfer G, Shastri S, Weber I, Glaubitz C, Mäntele W, Wachtveitl J (2011) Low temperature FTIR spectroscopy provides new insights in the pH-dependent proton pathway of proteorhodopsin. Biochim Biophys Acta Bioenerg 1807(12):1583–1590
Wakelin SA, Gupta VVSR, Forrester ST (2010) Regional and local factors affecting diversity abundance and activity of free-living N2-fixing bacteria in Australian agricultural soils. Pedobiol 53:391–399
Walker LR, del Moral R (2003) Primary succession and ecosystem rehabilitation. Cambridge University Press, Cambridge 442 pp
Wang J, Zhang D, Zhang L, Li J, Raza W, Huang Q, Shen Q (2016) Temporal variation of diazotrophic community abundance and structure in surface and subsoil under four fertilization regimes during a wheat growing season. Agric Ecosyst Environ 216:116–124
Ward JB, Berkeley RCW (1980) The microbial cell surface and adhesion. In: Berkeley RCW, Lynch JM, Melling J, Rutter PR, Vincent B (eds) Microbial adhesion to surfaces. Ellis Horwood Ltd, Chichester, pp 47–66
Watanabe I, Barraquio WL, De Guzman MR, Cabrera DA (1979) Nitrogen-fixing (acetylene reduction) activity and population of aerobic heterotrophic nitrogen fixing bacteria associated with wetland rice. Appl Environ Microbiol 37:813–819
Wolters V (1991) Biological processes in two beech forest soils treated with simulated acid rain—a laboratory experiment with Isotoma tigrina (Insecta Collembola). Soil Biol Biochem 23:381–390
Wu J, Brookes PC (2005) The proportional mineralization of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol Biochem 37:507–515
Yeager CM, Kornosky JL, Housman DC, Grote EE, Belnap J, Kuske CR (2004) Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado plateau and Chihuahuan desert. Appl Environ Microbiol 70:973–983
Young JPW (1996) Phylogeny and taxonomy of rhizobia. Plant Soil 186:45–52
Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554
Acknowledgements
The Head, Department of Botany, Coordinator CAS and DST-FIST programs, Department of Botany, BHU, Varanasi, India, is gratefully acknowledged for providing laboratory facilities. Central Instrumentation Laboratory, Department of Chemistry, Banaras Hindu University is acknowledged for FT-IR Analysis.
Funding
This study has been supported by the Council of Scientific and Industrial Research, New Delhi, India [38(1316)/12/EMR-II]. MS is also thankful to UGC, New Delhi, for providing financial assistance in the form of fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Srivastava, M., Mishra, A.K. Comparative responses of diazotrophic abundance and community structure to the chemical composition of paddy soil. Environ Sci Pollut Res 25, 399–412 (2018). https://doi.org/10.1007/s11356-017-0375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0375-6