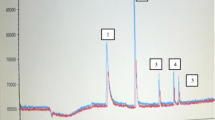
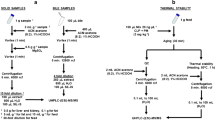
Abstract
Pharmaceuticals such as nonsteroidal anti-inflammatory drugs (NSAIDs) and lipid regulators are being repeatedly detected at low concentrations (pg · mL−1–ng · mL−1) in the environment. A large fraction of these compounds are ionizable. Ionized compounds show different physico-chemical properties and environmental behavior in comparison to their neutral analogs; as a consequence, the quantification methods currently available, based on the neutral molecules, might not be suitable to detect the corresponding ionized compounds. To overcome this problem, we developed a specific analytical method to quantify NSAIDs and lipid regulators (i.e., ibuprofen, diclofenac, naproxen, and clofibric acid) and their ionized compounds. This method is based on three steps: (1) the extraction of the organic compounds with an organic solvent assisted with an ultrasonic probe, (2) the cleaning of the extracts with a dispersive SPE with C18, and (3) the determination of the chemical compounds by GC-MS (prior derivatization of the analytes). We demonstrated that the proposed method can successfully quantify the pharmaceuticals and their ionized compounds in aqueous samples, lumpfish eggs, and zebrafish eleutheroembryos. Additionally, it allows the extraction and the cleanup of extracts from small samples (0.010 g of wet weight in pools of 20 larvae) and complex matrixes (due to high lipid content) and can be used as a basis for bioaccumulation assays performed with zebrafish eleutheroembryos in alternative to OECD test 305.


Similar content being viewed by others
References
Arnot JA, Gobas FAPC (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Env Rev 14(4):257–297
Arnot JA, Mackay D, Parkerton TF, Bonnell M (2008) A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11):2263–2270
Arroyo D, Ortiz MC, Sarabia LA (2011) Optimization of the derivatization reaction and the solid-phase microextraction conditions using a D-optimal design and three-way calibration in the determination of non-steroidal anti-inflammatory drugs in bovine milk by gas chromatography–mass spectrometry. J Chromatogr A 1218:4487–4497
Azzouz A, Ballesteros E (2015) Multiresidue method for the determination of pharmacologically active substances in egg and honey using a continuous solid-phase extraction system and gas chromatography–mass spectrometry. Food Chem 178:63–69
Azzouz A, Souhail B, Ballesteros E (2010) Continuous solid-phase extraction and gas chromatography –mass spectrometry determination of pharmaceuticals and hormones in water samples. J Chromatogr A 1217:2956–2963
Biterrmann K, Spycher S, Endo S, Pohler L, Huniar U, Goss KU, Klamt A (2014) Prediction of phospholipide-water partition coefficients of ionic organic chemicals using the mechanistic model COSMOmic. J Phys Chem B 110:14833–14842
Bittermann K, Spycher S, Goss KU (2016) Comparison of different models predicting the phospholipidmembrane water partition coefficients of charged compounds. Chemosphere 144:382–391
Brown JN, Paxéus N, Förlin L, Larsson DGL (2007) Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ Toxicol Phar 24:267–274
Brozinski JM, Lahti M, Meierjohann A, Oikari A, Kronberg L (2013) The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ Sci Technol 47:342–348
Caban M, Lis E, Kumirska J, Stepnowski P (2015) Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci Total Environ 538:402–411
Coimbra AM, Peixoto MJ, Coelho I, Lacerda R, Carvalho AP, Gesto M, Lyssimachou A, Lima D, Soares J, André A, Capitão A, Castro LFC, Santos MM (2015) Chronic effects of clofibric acid in zebrafish (Danio rerio): a multigenerational study. Aquat Toxicol 160:76–86
Corcoran J, Winter MJ, Tyler CR (2010) Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit Rev Toxicol 40:287–304
Corcoran J, Winter MJ, Lange A, Cumming R, Owen SF, Tyler CR (2015) Effects of the lipid regulating drug clofibric acid on PPAR-regulated gene transcript levels in common carp (Cyprinus carpio) at pharmacological and environmental exposure levels. Aquat Toxicol 161:127–137
Debska J, Kot-Wasik A, Namiesnik J (2005) Determination of nonsteroidal anti-inflammatory drugs in water samples using liquid chromatography coupled with diode-array detector and mass spectrometry. J Sep Sci 28:2419–2426
Dubreil-Chéneau E, Pirotais Y, Bessiral M, Roudaut B, Verdon E (2011) Development and validation of a confirmatory method for the determination of 12 non steroidal anti-inflammatory drugs in milk using liquid chromatography–tandem mass spectrometry. J Chromatogr A 1218:6292–6301
El-Amrani S, Pena-Abaurrea M, Sanz-Landaluze J, Ramos L, Guinea J, Cámara C (2012) Bioconcentration of pesticides in zebrafish eleutheroembryos (Danio rerio). Sci Total Environ 425:184–190
EPI Suite BCFWIN™ (2012) Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11 or insert version used. United States Environmental Protection Agency, Washington, DC, USA
Erickson RJ, Mckim JM, Lien GJ, Hoffman AD, Batterman SL (2006) Uptake and elimination of ionizable organic chemicals at fish gills: II. Observed and predicted effects of pH, alkalinity, and chemical properties. Environ Toxicol Chem 25:1522–1532
European Chemicals Agency (2014) Guidance on Information requirements and chemical safety assessment. Endpoint specific guidance, chapters R.7a. European Chemicals Agency, Helsinki, Finland
European Parliament and of the Council (2010) Legislation for the protection of animals used for scientific purposes (Directive 2010/63/EU)
Franco A, Ferranti A, Davidsen C, Trapp S (2010) An unexpected challenge: ionizable compounds in the REACH chemical space. Int J Life Cycle Assess 15:321–325
Gentili A, Caretti F, Bellante S, Mainero Rocca L, Curini R, Venditti A (2012) Development and validation of two multiresidue liquid chromatography tandem mass spectrometry methods based on a versatile extraction procedure for isolating non-steroidal anti-inflammatory drugs from bovine milk and muscle tissue. Anal Bional Chem 404:1375–1388
German Federal Environment Agency (UBA) (2015) REACH Compliance: Data Availability of REACH Registrations, Project No. (FKZ) 3714 67 4200. Report No. (UBA-FB) 002111
Gonzalo-Lumbreras R, Sanz-Landaluze J, Guinea J, Cámara C (2012) Miniaturized extraction methods of triclosan from aqueous and fish roe samples. Bioconcentration studies in zebrafish larvae (Danio rerio). Anal Bioanal Chem 403:927–937
Grueiro Noche G, Fernández Laespada ME, Pérez Pavón JL, Moreno Cordero B, Muniategui Lorenzo S (2011) In situ aqueous derivatization and determination of non-steroidal anti-inflammatory drugs by salting-out-assisted liquid–liquid extraction and gas chromatography–mass spectrometry. J Chromatogr A 1218:6240–6247
Guitart C, Readman JW (2010) Critical evaluation of the determination of pharmaceuticals, personal care products, phenolic endocrine disrupters and faecal steroids by GC/MS and PTV-GC/MS in environmental waters. Anal Chim Acta 658:32–40
Hilal SH, Saravanaraj AN, Whiteside T, Carreira LA (2007) Calculating physical properties of organic compounds for environmental modeling from molecular structure. J Comput Aided Mol Des 21:693–708
Hoon Lee C, Shin Y, Woo Nam M, Yeong KM, Lee J (2014) A new analytical method to determine non-steroidal anti-inflammatory drugs in surface water using in situ derivatization combined with ultrasound-assisted emulsification microextraction followed by gas chromatography–mass spectrometry. Talanta 129:552–559
Hu T, Peng T, Li X, Chen D, Dai H, Deng X, Yue Z, Wang G, Shen J, Xia X, Ding S, Zhou Y, Zhu A, Jiang H (2012) Simultaneous determination of thirty non-steroidal anti-inflammatory drug residues in swine muscle by ultra-high-performance liquid chromatography with tandem mass spectrometry. J Chromatogr A 1219:104–113
Huerta B, Jakimska A, Gros M, Rodríguez-Mozaz S, Barceló D (2013) Analysis of multi-class pharmaceuticals in fish tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1288:63–72
Jedziniak P, Szprengier-Juszkiewicz T, Olejnik M, Zmudzki J (2010) Determination of non-steroidal anti-inflammatory drugs residues in animal muscles by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 672:85–92
Kang J, Park S, Park H, Gedi V, So B, Lee K (2014) Multiresidue determination of ten nonsteroidal anti-inflammatory drugs in bovine, porcine, and chicken liver tissues by HPLC-MS/MS. Appl Biochem Biotechnol 174:1–5
Lahti M, Brozinski J-M, Jylhä A, Kronberg L, Oikari A (2011) Uptake from water, biotransformation, and biliary excretion of pharmaceuticals by rainbow trout. Environ Toxicol Chem 30:403–1411
Larsson E, Al-Hamimi S, Áke Jönsson J (2014) Behaviour of nonsteroidal anti-inflammatory drugs and eight of their metabolites during wastewater treatment studied by hollow fibre liquid phase microextraction and liquid chromatography mass spectrometry. Sci Total Environ 485–486:300–308
Li J, Xu L, Shi Z, Hu M (2015) A novel two-dimensional liquid chromatographic system for the online toxicity prediction of pharmaceuticals and related substances. J Hazard Mater 293:15–20
Macia A, Borrul F, Calul M, Aguilar C (2007) Capillary electrophoresis for the analysis of non-steroidal anti-inflammatory drugs. Trends Anals Chem 26(2):133–153
Matamoros V, Calderón-Preciado D, Domínguez C, Bayona JM (2012) Analytical procedures for the determination of emerging organic contaminants in plant material: a review. Anal Chim Acta 722:8–20
Migowska N, Caban M, Stepnowski P, Kumirska J (2012) Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Sci Total Environ 441:77–88
Nallani GC, Paulos PM, Constantine LA, Venables BJ, Huggett DB (2011) Bioconcentration of ibuprofen in fathead minnow (Pimephales promelas) and channel catfish (Ictalurus punctatus). Chemosphere 84:1371–1377
Nunes B, Carvalho F, Guilhermino L (2005) Acute toxicity of widely used pharmaceuticals in aquatic species: Gambusia holbrooki, Artemia parthenogenetica and Tetraselmis chuii. Ecotox Environ Safe 61:413–419
OECD 2012. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure, OECD Guidelines for the Testing of Chemicals, Section 3, O.P., 2012. OECD guidelines for testing of chemicals. Bioaccumulation in Fish : Aqueous and Dietary Exposure doi:10.1787/2074577x
Ortiz de García S, Pinto Pinto G, García-Encina PA, Irusta Mata R (2013) Ranking of concern, based on environmental indexes, for pharmaceutical and personal care products: an application to the Spanish case. J Environ Manag 129:384–397
Paíga P, Lolic A, Hellebuyck F, Santos Lúcia HMLM, Correia M, Delerue-Matos C (2015) Development of a SPE–UHPLC–MS/MS methodology for thedetermination of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawater. J Pharmaceut Biomed 106:61–70
Praskova E, Voslarova E, Siroka Z, Plhalova L, Mocova S (2011) Assessment of diclofenac LC50 reference values in juvenile and embryonic stages of the zebrafish (Danio rerio). Pol J Vet Sci 14(4):545–549
Sadat Hasheiminasab K, Reza Fakhari A, Shahsavani A, Ahmar H (2013) A new method for the enhancement of electromembrane extraction efficiency using carbon nanotube reinforced hollow fiber for the determination of acidic drugs in spiked plasma, urine, breast milk and wastewater samples. J Chromatogr A 1285:1–6
Sagristá E, Larsson E, Ezoddin M, Hidalgo M, Salvadó V, Jönsson JA (2010) Determination of non-steroidal anti-inflammatory drugs in sewage sludge by direct hollow fiber supported liquid membrane extraction and liquid chromatography–mass spectrometry. J Chromatogr A 1217:6153–6158
Sanz-Landaluze J, Pena-Abaurrea M, Muñoz-Olivas R, Cámara C, Ramos L (2015) Zebrafish (Danio rerio) eleutheroembryo-based procedure for assessing bioaccumulation. Environ Sci Technol 49:1860–1869
Saravanan M, Karthika S, Malarvizhi A, Ramesh M (2011) Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J Hazard Mater 195:188–194
Saravanan M, Usha Devi K, Malarvizhi A, Ramesh M (2012) Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp. Environ Toxicol Pharm 34:14–22
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol 68:141–150
Sousa MA, Gonçalves C, Cunha E, Hajšlová J, Alpendurada MF (2011) Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal Bioanal Chem 399:807–822
Subedi B, Mottaleb MA, Chambliss CK, Usenko S (2011) Simultaneous analysis of select pharmaceuticals and personal care products in fish tissue using pressurized liquid extraction combined with silica gel cleanup. J Chromatogr A 1218:6278–6284
Tanoue R, Nomiyama K, Nakamura H, Hayashi T, Kim JW, Isobe T, Shinohara R, Tanabe S (2014) Simultaneous determination of polar pharmaceuticals and personal care products in biological organs and tissues. J Chromatogr A 1355:193–205
Wang J, Gardinali PR (2013) Uptake and depuration of pharmaceuticals in reclaimed water by mosquito fish (Gambusia holbrooki): a worst-case, multiple-exposure scenario. Environ Toxicol Chem 32(8):1752–1758
Westerfield MA (2007) Guide for the Laboratory Use of Zebrafish (Danio Rerio), 5th Edition. University of Oregon Press, Eugene, USA.
Xu J, Wu L, Chen W, Chang AC (2008) Simultaneous determination of pharmaceuticals, endocrine disrupting compounds and hormone in soils by gas chromatography–mass spectrometry. J Chromatogr A 1202:189–195
Yu Y, Wu L (2011) Comparison of four extraction methods for the analysis of pharmaceuticals in wastewater. J Chromatogr A 1218:2483–2489
Yu Y, Wu L (2012) Analysis of endocrine disrupting compounds, pharmaceuticals and personal care products in sewage sludge by gas chromatography–mass spectrometry. Talanta 89:258–263
Zenker A, Cicero MR, Prestinaci F, Bottoni P, Carere M (2014) Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J Environ Manage 133:378–387
Zhang H, Du Z, Ji Y, Mei M (2013) Simultaneous trace determination of acidic non-steroidal anti-inflammatory drugs in purified water, tap water, juice, soda and energy drink by hollow fiber-based liquid-phase micro extraction and ultra-high pressure liquid chromatography coupled to tandem mass spectrometry. Talanta 109:177–184
Acknowledgments
The authors want to thank the financial support of this work by the Ministry of Economy and Competitivity (Project SAFEFOOD, grant code CTQ2014-54801-C2-1-R), Comunidad Autónoma of Madrid (Project AVANSECAL, code S2013/ABI-3028) and Universidad Complutense for supporting the research group “Determinación de Trazas y Especiación.” Noemí Molina thanks the Spanish Ministry of Education for the FPU predoctoral fellowship (Ref. AP2010-0740). We are grateful to AZTI (Bizkaia, Spain) for providing the eleutheroembryo samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Fig. S1 Analyte areas obtained using different derivatization time for a solution of four compounds (CFB, IBU, NP, DC) at 1 μg · mL−1. Table S1 Recoveries and %RSD obtained for the four pharmaceuticals in an intra and inter-day study at two different concentrations for the three different matrices. (DOC 88 kb)
Rights and permissions
About this article
Cite this article
Molina-Fernandez, N., Perez-Conde, C., Rainieri, S. et al. Method for quantifying NSAIDs and clofibric acid in aqueous samples, lumpfish (Cyclopterus lumpus) roe, and zebrafish (Danio rerio) eleutheroembryos and evaluation of their bioconcentration in zebrafish eleutheroembryos. Environ Sci Pollut Res 24, 10907–10918 (2017). https://doi.org/10.1007/s11356-016-6671-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6671-8