Abstract
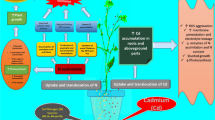
This study, based on a greenhouse pot culture experiment conducted with 15-day-old rapeseed (Brassica campestris L. cv. Pusa Gold; family Brassicaceae) and moong bean (Vigna radiata L. Wilczek cv. Pusa Ratna; family Fabaceae) plants treated with cadmium (Cd) concentrations (0, 50, and 100 mg kg−1 soil), investigates their potential for Cd accumulation and tolerance, and dissects the underlying basic physiological/biochemical mechanisms. In both species, plant dry mass decreased, while Cd concentration of both root and shoot increased with increase in soil Cd. Roots harbored a higher amount of Cd (vs. shoot) in B. campestris, while the reverse applied to V. radiata. By comparison, root Cd concentration was higher in B. campestris than in V. radiata. The high Cd concentrations in B. campestris roots and V. radiata shoots led to significant elevation in oxidative indices, as measured in terms of electrolyte leakage, H2O2 content, and lipid peroxidation. Both plants displayed differential adaptation strategies to counteract the Cd burden-caused anomalies in their roots and shoots. In B. campestris, increasing Cd burden led to a significantly decreased reduced glutathione (GSH) content but a significant increase in activities of GSH reductase (GR), GSH peroxidase (GPX), and GSH sulfotransferase (GST). However, in V. radiata, increasing Cd burden caused significant increase in GSH content and GR activity, but a significant decline in activities of GPX and GST. Cross talks on Cd burden of tissues and the adapted Cd tolerance strategies against Cd burden-accrued toxicity indicated that B. campestris and V. radiata are good Cd stabilizer and Cd extractor, respectively, wherein a fine tuning among the major components (GR, GPX, GST, GSH) of the GSH redox system helped the plants to counteract differentially the Cd load-induced anomalies in tissues. On the whole, the physiological/biochemical characterization of the B. campestris and V. radiata responses to varying Cd concentrations can be of great help in elaborating the innovative plant-based remediation technologies for metal/metalloid-contaminated sites.





Similar content being viewed by others
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Anderson ME (1985) Determination of glutathione and glutathione disulphides in biological samples. Method Enzymol 113:548–570
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008a) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul 54:271–279
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008b) Ontogenic variation in response of Brassica campestris L. to cadmium toxicity. J Plant Interac 3:189–198
Anjum NA, Umar S, Ahmad A, Iqbal M (2008c) Responses of components of antioxidant system in moongbean [Vigna radiata (L.) Wilczek] genotypes to cadmium stress. Commun Soil Sci Plant Anal 39:2469–2483
Anjum NA, Umar S, Chan MT (2010) Ascorbate-glutathione pathway and stress tolerance in plants, 1st edn. Springer, Dordrecht
Anjum NA, Ahamd I, Mohmood I, Pacheco M, Duarte AC et al (2012a) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Umar S, Ahmad A (2012b) Oxidative stress in plants: causes, consequences and tolerance. IK International, New Delhi
Anjum NA, Ahmad I, Pereira ME, Duarte AC et al (2012c) The plant family Brassicaceae: contribution towards phytoremediation, 1st edn. Springer (Science + Business Media), Dordrecht
Anjum NA, Ahmad I, Rodrigues SM, Henriques B et al (2013a) Eriophorum angustifolium and Lolium perenne metabolic adaptations to metals- and metalloids-induced anomalies in the vicinity of a chemical industrial complex. Environ Sci Pollut Res 20:568–581
Anjum NA, Singh N, Singh MK, Shah ZA et al (2013b) Single-bilayer graphene oxide sheet tolerance and glutathione redox system significance assessment in faba bean (Vicia faba L.). J Nanopart Res 15:1770
Anjum NA, Duarte AC, Pereira E, Ahmad I (2014) Oxidative stress status, antioxidant metabolism and polypeptide patterns in Juncus maritimus shoots exhibiting differential mercury-burdens in Ria de Aveiro coastal lagoon (Portugal). Environ Sci Pollut Res. doi:10.1007/s11356-014
Clemens S, Palmgren MG, Kraemer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trend Plant Sci 7:309–315
Cuypers A, Keunen E, Bohler S, Jozefczak M et al (2012) Cadmium and copper stress induce a cellular oxidative challenge leading to damage versus signalling. In: Gupta DKG, Sandalio LM (eds) Metal toxicity in plants: perception, signaling and remediation. Springer, Berlin, pp 65–90
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signal Behav 6:215–222
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Gill SS, Anjum NA, Hasanuzzaman M, Gill R et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212
International Agency for Research on Cancer (IARC) (1993) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 58. Lyon: International Agency for Research on Cancer; 1993. Beryllium, cadmium, mercury and exposures in the glass manufacturing industry; pp. 41-117
Irfan M, Hayat S, Ahmad A, Alyemeni MN (2013) Soil cadmium enrichment: allocation and plant physiological manifestations. Saudi J Biol Sci 20:1–10
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quences ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13
Mohamed AA, Castagna A, Ranieri A, Sanita di Toppi L (2012) Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem 57:15–22
Polle A, Schützendübel A (2003) Heavy metal signalling in plants: linking cellular and organismic responses. In: Hirt H, Shinozaki K (eds) Plant responses to abiotic stress. Springer, Berlin, pp 187–215
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Satarug S, Baker JR, Reilly PEB, Moore MR, Williams DJ (2002) Cadmium levels in the lung, liver, kidney cortex and urine samples from Australians without occupational exposure to metals. Arch Environ Health 57:69–77
United Nations Environment Programme (2008) Draft final review of scientific information on cadmium. http://www.chem.unep.ch/pb_and_cd/SR/Draft_final_reviews_Nov2008.htm (accessed on 4 March 2014)
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land—a review. Environ Chem Lett 8:1–17
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wahid A, Ghani A, Ali I, Ashraf MY (2007) Effect of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J Agron Crop Sci 193:357–365
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
WHO (2007) Health risks of heavy metals from long-range transboundary air pollution. World Health Organization 2007. WHO Regional Office for Europe, Copenhagen, Denmark
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Zaidi A, Wani PA, Khan MS (2012) Toxicity of heavy metals to legumes and bioremediation. Springer, Dordrecht
Acknowledgements
Financial assistance received from Hamdard National Foundation (HNF), New Delhi, India (DSW/HNF-18/2006) is gratefully acknowledged. NAA also thanks the FCT (Govt. of Portugal) for providing post-doctoral grants (FRH/BPD/64690/2009; SFRH/BPD/84671/2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Anjum, N.A., Umar, S. & Iqbal, M. Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants—implications for phytoremediation. Environ Sci Pollut Res 21, 10286–10293 (2014). https://doi.org/10.1007/s11356-014-2889-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2889-5