Abstract
Introduction and methods
This study investigated the remediation of cadmium-polluted soil using a combination of stainless steel slag and ammonium humate. These remedial agents were added to an artificially polluted garden soil to inhabit cadmium toxicity in soil by changing the physical and chemical properties of soil in a pot experiment.
Results and conclusions
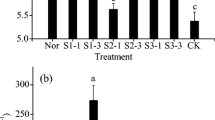
The results showed that the co-application of ammonium humate and stainless steel slag significantly decreased the total and available soil cadmium concentrations, with maximum decreases of 16.30% and 58.04%, respectively. The co-application of an adequate dose of these remedial agents can significantly increase soil pH. The soil organic matter and cation exchange capacity, as well as the amount of soil aggregates, were also significantly increased by the addition ammonium humate, but not stainless steel slag.




Similar content being viewed by others
References
Ali I, Gupta VK (2006) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ayuso M, Hernandez T, Garcia C, Pascual JA (1996) Stimulation of barley growth and nutrient absorption by humic substances originating from various organic materials. Bioresource Technol 57:251–257
Castaldi P, Santona L, Melis P (2005) Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60:365–371
Ceppi SB, Velasco MI, De Pauli CP (1999) Differential scanning potentiometry: surface charge development and apparent dissociation constant of natural humic acids. Talanta 50:157–1063
Chen Y, Aviad T (1990) Effect of humic substances on plant growth. In: MacCarthy P (ed) Humic substances in soil and crop sciences: selected readings. ASA-SSSA, Madison, pp 161–186
Chen CL, Wang XK (2006) Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind Eng Chem Res 45:9144–9149
Clinton PW, Newman RH, Allen RB (1995) Immobilization of 15N in forest litter studied by 15N CPMAS NMR spectroscopy. Eur J Soil Sci 46:551–556
Deng THB, Gu HH, Qiu RL (2011) Effects of steel slag application on multi-metal contaminated soil and heavy metal uptake of rice. J Agro-Environ Sci 30:455–460
García-Gil JC, Ceppi SB, Velasco MI, Polo A, Senesi N (2004) Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 121:135–142
Ghabbour EA, Shaker M, El-Toukhy A, Abid IM, Davies G (2006) Thermodynamics of metal cation binding by a solid soil-derived humic acid: binding of Fe(III), Pb(II), and Cu(II). Chemosphere 63:477–483
Giannis A, Gidarakos E, Skouta A (2007) Application of sodium dodecyl sulfate and humic acid as surfactants on electrokinetic remediation of cadmium-contaminated soil. Desalination 211:249–260
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interf Sci 271:321–328
Gupta VK, Rastogi A (2008a) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloid Surf B 64:170–178
Gupta VK, Rastogi A (2008b) Sorption and desorption studies of chromium(VI) from nonviable Cyanobacterium nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2008c) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash—a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Mohan D, Sharma S (1998) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environment 19:129–136
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Premvir S, Nafisur R (2004) Adsorption behavior of Hg(II), Pb(II), andCd(II) from aqueous solution on Duolite C-433: asynthetic resin. J Colloid Interf Sci 275:398–402
Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Ali I, Saini VK (2007a) Adsorption studies on the removal of vertigo blue 49 and orange DNA 13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interf Sci 315:87–93
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007b) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interf Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007c) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas MML (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Env Sci Tec 39:783–842
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interf Sci 342:135–141
Hrudey SE, Chen W, Rousseau CG (1995) Bioavailability in environmental risk assessment. Lewis Pub, Boca Raton, FL
Karaca A, Naseby DC, Lynch JM (2002) Effect of cadmium contamination with sewage sludge and phosphate fertiliser amendments on soil enzyme activities, microbial structure and available cadmium. Biol Ferti Soils 35:428–434
Kim DH, Shin MC, Choia HD, Seo CI, Baek K (2008) Removal mechanisms of copper using steel-making slag: adsorption and precipitation. Desalination 223:283–289
Li P, Wang XX, Zhang TL, Zhou DM, He YQ (2008) Effects of several amendments on rice growth and uptake of copper and cadmium from a contaminated soil. J Environ Sci 20:449–155
Li H, Shi WY, Shao HB, Shao MA (2009) The remediation of the lead-polluted garden soil by natural zeolite. J Hazard Mater 169:1106–1111
Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liu SY, Gao J, Yang YJ, Yang YC, Ye ZX (2010) Adsorption intrinsic kinetics and isotherms of lead ions on steel slag. J Hazard Mater 173:558–562
McGrath SP, Cegarra J (1992) Chemical extractability of heavy metals during and after long-term applications of sewage sludge to soil. J Soil Sci 43:313–321
Moreno JL, Hernández T, Pérez A, García C (2002) Toxicity of cadmium to soil microbial activity: effect of sewage sludge addition to soil on the ecological dose. Appl Soil Ecol 21:149–158
Moreno JL, García C, Hernández MT (2003) Toxic effect of cadmium and nickel on soil enzymes and the influence of adding sewage sludge. Eur J Soil Sci 54:377–386
Moreno JL, Sánchez-Marín A, Hernández MT, Garcia C (2006) Effect of cadmium on microbial activity and a ryegrass crop in two semiarid soil. Environ Manag 37:626–633
Nisar A, Mir S (1989) Lignitic coal utilization in the form of HA as fertilizer and soil conditioner. Sci Tech Develop 8:23–26
Pehlivan E, Arslan G (2006) Comparison of adsorption capacity of young brown coals and humic acids prepared from different coal mines in Anatolia. J Hazard Mater B 138:401–408
Sharif M, Khattak RA, Sarir MS (2002) Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun Soil Sci Plant Anal 33:3567–3580
Shi WY, Shao HB, Li H, Shao MA, Du S (2009a) Co-remediation of the lead-polluted garden soil by exogenous natural zeolite and humic acids. J Hazard Mater 167:136–140
Shi WY, Shao HB, Li H, Shao MA, Du S (2009b) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170:1–6
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Enviorn Eng-ASCE 123:461–468
Tang XY, Zhu YG, Bei YS, Duan J, Tang L (2006) The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ Int 32:682–689
Thakur SK, Tomar NK, Pandeya SB (2006) Influence of phosphate on cadmium sorption by calcium carbonate. Geoderma 130:240–249
Tordoff GM, Baker AJM (2000) Current approaches to the revetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Wang X, Cai QS (2006) Steel slag as an iron fertilizer for corn growth and soil improvement in a pot experiment. Pedosphere 16:519–524
Wang XK, Chen CL, Du JZ, Tan XL, Xu D, Yu SM (2005) Effect of pH and aging time on the kinetic dissociation of 243Am(III) from humic acid-coated γ-Al2O3: a chelating resin exchange study. Environ Sci Technol 39:7084–7088
Acknowledgments
This research was supported by the Grand Science and Technology Special Project of Shanxi Province (20111101016); Science & Technology Key Program of Shanxi Province (20090311072); the Key Research Program of State Key Laboratory of Loess and Quaternary Geology; Initial Funding from the Institute of Earth Environment, Chinese Academy of Sciences; the West Light Foundation of the Chinese Academy of Sciences; and the Technology Center of the Shanxi Taigang Stainless Steel Co. Ltd.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Zhuo, L., Li, H., Cheng, F. et al. Co-remediation of cadmium-polluted soil using stainless steel slag and ammonium humate. Environ Sci Pollut Res 19, 2842–2848 (2012). https://doi.org/10.1007/s11356-012-0790-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0790-7