Abstract
Fipronil has been associated with neurotoxicity, carcinogenicity, endocrine disruption, persistence in soil, and low uptake by plants and is a potential groundwater contaminant. Fipronil degradation by silver nanoparticles (AgNPs) from medicinal plant extracts was investigated in spiked water. Also, remediation capacity of soil contaminated by fipronil under the combined application of green AgNPs and phytoremediation was investigated. Brassica-AgNps, Ipomoea-AgNps, Camellia-AgNps, and Plantago-AgNps in water solution significantly reduced fipronil residues by 95.45, 90.15, 63.65, and 63.48%) during 2 days of treatment as compared with 18.42% in untreated water without AgNps. Fipronil amide and fipronil-desulfenyl metabolites were detected in water under the influence of AgNps. The contribution of Brassica-AgNps, Plantago-AgNps, Ipomoea-AgNps, and Camellia-AgNps to the dissipation of fipronil in the soil were 68.8, 54.64, 43.75, and 30.99%, respectively, compared with 10.14% by Plantago major alone through 6 days. Low uptake and translocation of fipronil by P. major roots and leaves were seen in flooded soil alone or under the influence of AgNps within 6 days of treatment. However, the resulting fipronil amide product accumulates in large quantities in P. major roots and leaves. These results show that AgNps and P. major play a major role for the remediation of fipronil contaminated water and in flooded soil, while P. major played an important role for remediation the polar break product, fipronil-amide as phytoremediation.
Similar content being viewed by others
1 Introduction
A phenyl pyrazole insecticide, Fipronil, is a broad-spectrum foliar and soil insect in agricultural, forestry, and pastoral zones that has been approved for use on more than100 different crops in 70 countries (Alyokhin et al. 2008; Okumura et al. 2016). Fipronil has been associated with neurotoxicity, endocrine disruption and carcinogenicity and is a potential groundwater contaminant (Ohi et al. 2004; Raquel et al. 2011; Gan et al. 2012), so there has been an increasing concern about the environmental pollution and human health effects associated with fipronil use (Gunasekara et al. 2007; Lee et al. 2010). Fipronil degradation in the soil was detected by exposure to sunlight at the surface to yield fipronil-desulfinyl, oxidation near the surface to produce fipronil-sulfone, hydrolysis through the upper layer to produce fipronil amide, and reductive processes below the surface which lead to the production of fipronil-sulfide (Pei et al. 2004; Raveton et al. 2007). The t1/2s of fipronil and its products in soil indicate that they are persistent; degradation typically ranges from 111 to 350 days, depending upon soil conditions (Gunasekara. et al. 2007). Moreover, U.S. EPA (1996) suggested that fipronil has a low to slight mobility in soil (log Kow 3.9–4.1); this would result in a low potential for groundwater contamination, except for the very water-soluble fipronil amide. Most of the degradation products of fipronil were found to not move below 10 cm (U.S. EPA 1996). The study also noted that leaching of fipronil amide coincided with rainfall (Bobe et al. 1998). Studies of metabolism after soil incorporation carried out with [14C]fipronil on maize, sunflower, and sugar beet show an uptake of about 5% (Dieckmann et al., 2010). Analysis of extracts of maize forage samples revealed fipronil, fipronil sulfone, and fipronil amide as the major metabolites; fipronil carboxylic acid was also found. Also, in pollen and honey, fipronil, fipronil desulfinyl, and fipronil sulfone have been detected (Bonmatin et al. 2007; Chauzat et al. 2011). Nanoremediation means the utilization of highly reactive nanoscale materials for the elimination of pollutants and the purification of water, air, or ground bodies through catalytic processes or chemical reduction (Wang et al. 2014).
Green synthesis of FeNPs and silver nanoparticles (AgNPs) involves the use of extracts of natural plants as reducing agents to reduce Fe (II) or Fe (III) or Ag salts to form FeNPs or AgNPs. This strategy is simple, eco-friendly, and cost effective. Moreover, the nanoparticles formed in this way are more stable (Huo et al. 2017). Some of the plant extracts used in the synthesis of silver nanoparticles include Cinnamomum camphora leaf extract (Huang et al. 2007), Camellia sinensis (Green tea) leaf extract (Vilchis-Nestor et al. 2008), Aloe vera extract (Chandra et al. 2006), Allium sativum (garlic) extract (Von White et al. 2012), Capsicum annum (pepper) leaf extract (Li et al. 2007), Mukia maderaspatana root extract (Subha et al. 2016), and Citrullus lanatus (watermelon) fruit rind extract (Ndikau et al. 2017).
Low availability of fipronil in soil and low uptake by plants such as maize, sunflower, and sugar (Dieckmann et al., 2010) may result in lower removal by phytoremediation, which needed to be assisted by green nanotechnology including silver nanoparticles with degradation capacity suggested as a strategy for improving remediation efficiency of fipronil. Approximately, no researches focus on the remediation efficiency of co-contaminated soil under the combined application of green nanotechnology and plant.
Therefore, the objectives of this study were to (1) investigate the effects of silver nanoparticles from medicinal plant extracts on the degradation of fipronil in water and (2) evaluate the contribution of using silver nanoparticles as green nanotechnology and Plantago major for enhancing the availability and uptake and for speeding up the dissipation of fipronil-contaminated soil.
2 Materials and Methods
2.1 Material Used
Fresh Ipomoea carnea leaves were obtained from irrigation channels at Zagazig district, Sharkia governorate, Egypt, while fresh Plantago major leaves were obtained from meadow lands in Zagazig University, Zagazig, Sharkia governorate, Egypt. Also, dry green tea (Camellia sinensis) leaves and dry seeds of Brassica alba were obtained from Cairo Tea Factory, 6th Of October City, Egypt, and National Research Center, Dokki, Egypt, respectively.
Silver nitrate (99.9%) was obtained from Sigma-Aldrich.
2.2 Synthesis of Silver Nanoparticles by Medicinal Plant Extracts
The leaf extract was prepared as follows, 20 g of each fresh Ipomoea carnea leaves, and Plantago major leaves were cut and washed with deionized water and chopped into small pieces. Also, 20 g of each dry green tea (Camellia sinensis) leaves and dry seeds of Brassica alba were powdered, after which they were added to 100 ml of deionized sterile water and kept in a 100 °C water bath for 60 min. The extracts from leaves and seeds were then mixed with enough 1 mM silver nitrate to give the desired solution (0.5, 1, 2, 5, 10, and 20% v/v) and allowed to react at room temperature (Daniel et al. 2014).
2.3 UV-Visible Spectroscopy Characterization
The formation and stability of silver nanoparticles (AgNps) was done by using UV-vis spectrophotometer (Version 530). The absorption spectrum of reaction solutions were recorded at wavelengths ranging from 200 to 800 nm (Narayan and Park 2014).
2.4 Pesticide and Plant Materials
Fipronil 5% E.C., was obtained from the Central Agriculture Pesticide Laboratory, Agriculture Research Center, 7 Nadi EL Said St., Dokki, Giza, Egypt.
The common broadleaf plantain (Plantago major L.) were obtained as seedlings (9–12 cm in height with 4–6 leaves) in a phytoremediation experiment from meadow lands in Zagazig University, Zagazig, Sharkia governorate, Egypt.
2.5 Experimental Design
Two milliliters of silver nanoparticles (AgNps, 2%) was added to 98 ml of water containing 65 mg fipronil/L of water in a 250-ml Erlenmeyer flask. Three replicates were used for each AgNp and three flasks were prepared as a control without AgNps. The experiment was studied at room temperature (30 ± 2 °C). After 2 and 6 days, samples of water from exposed and controls were collected for extraction and determination of fipronil and degradation products.
Also, AgNps were used to enhance the degradation efficiency of fipronil-contaminated soil with Plantago major. The experiment used a pot assay; the pot-culture experiment was arranged in a randomized design that contained seven treatments: (1) uncontaminated soil with a P. major seedling, (2) fipronil-contaminated soil with no plants, (3) fipronil-contaminated soil with a P. major seedling, (4–7) fipronil-contaminated soil amended with AgNps (2.0%) from C. sinensis, B. alba, I. carnea, and P. major separately, with a P. major. A water solution of fipronil (65 mg/kg soil) was carefully added to the pots so as to avoid direct contact with the plant shoots. Two milliliters of each AgNps (2.0%) was diluted to 100 ml of water and amended to the soil (treatments, 4–7) until flooding (2 cm) to maintain an anaerobic condition, while treatments 2 and 3 were amended with water alone until flooding. Each treatment was replicated three times. In experiments (1, 3–7), each pot contained one seedling of P. major. The soil used in this experiment was collected from a plot at Aboutwala, Minya al Qamh, Sharqia, Egypt. The air-dried, sieved clay loam soil contained 2.0% organic matter, had a pH of 7.9, and had a cation exchange capacity of 42.12 cmol/kg; 400 g of soil was placed in each plastic pot. Soil was spiked with fipronil to get the initial concentration of 65 mg/kg dry weight soil. At 6 days exposure times, the plants were harvested from the treatment soils for analyses. Plant roots were rinsed in running tap water for 2 min and blotted dry. The plants were dissected into individual roots and leaves. Four grams of roots, 4 g of leaves, and 10 g of soil were analyzed for the determination of fipronil residues or the degradation products.
Extraction of Fipronil and Degradation Products from Water, Soil, and Plant
Acetonitrile was added to the liquid samples in a 1:1 ratio (v:v) and mixed well. A quantity of 1.0 mL of each mixture was filtered through a 0.45-μm nylon filter and 10 μL of the filtrate was injected into HPLC (Shuai et al. 2011). A volume of 20.0 mL of 100% acetonitrile was added to each soil sample and shaken for 2 h to extract fipronil and its metabolites from the soil. Samples were then allowed to settle and 4 mL of the supernatant was filtered through a 0.45-μm nylon filter. Ten microliters of the resulting filtrate was injected into HPLC (Shuai et al. 2011). Samples (4 g of each root or leaf) were put into 50-mL centrifuge tubes, then 5 mL aqueous solution of formic acid (0.1%) and 20 mL acetonitrile were added. After the tubes were vortexed for 2 min, NaCl (3 g) was put into each tube. Then, the samples were vortexed again for 1 min and centrifuged at 3800 rpm (2260 rcf) for 5 min. The extract (1 mL) was transferred into a 2-mL centrifuge tube with 50 mg PSA, and the tube was vortexed for 1 min. Afterwards, each tube was centrifuged at 10,000 rpm for 1 min, and the extract was filtered through a 0.22-μm nylon filter and10 μL of the resulting filtrate was injected into HPLC (Li et al. 2015).
HPLC Analysis
Samples (water, soil, root, and leaf extracts) were analyzed for the determination of fipronil and degradation products by high-performance liquid chromatography (HPLC). The operating parameters were as follows: a C-18 reversed-phase column was used (250 × 4.0 mm i.d.), mobile phase consisted of (85:15) acetonitrile-water with isocratic mode at a flow rate of 1.3 mL/min, and UV detection at 280 nm. The injection volume was 10 μL for quantitative analysis. The retention time of fipronil, fipronil amide, fipronil desulfenyl, and fipronil sulfone under the abovementioned conditions was 2.73, 1.90, 2.16, and 2.93 min. The recovery of fipronil was determined at two fortification levels (i.e., 0.1 and 0.5 mg/kg), for each matrix (root, leaf, and soil samples). No interfering peaks were observed in sample chromatograms under the selected conditions. The recoveries obtained for roots, leaves, and soil samples were in the range 88.0–90.5%, 86.4–89.0%, and 86.0–88.5%, respectively. The extraction efficiency of the analytical procedure was evaluated via recovery experiments conducted in triplicate using the fortified blank leaves and roots of P. major, water and soil samples at two different concentrations of fipronil, 0.1 and 0.5 mg/kg for soil and plant, 0.1 and 0.5 mg/L or water. The average recoveries obtained for water, root, leaf, and soil samples were in the range 92.8–95%, 89.0–91.2%, 87.4–88.23%, and 88.0–89.5%, respectively.
Statistical Analysis
Data were evaluated statistically by one-way ANOVA, and comparison of mean values (mean ± SD) was done by using Tukey’s honestly significant difference test at p ≤ 0.05. The CoStat 6.311 CoHort Statistical Software was used for the analysis.
3 Results and Discussion
3.1 UV-Visible Spectroscopic Analysis and Color Change
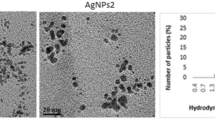
A color change to dark brown through 5 min was showed by mixing 0.5% leaf extract of Camellia sinensis with 1 mM silver nitrate solution, while a color change to reddish brown was showed by mixing plant extracts of Ipomoea carnea, Plantago major, and Brassica alba at 2% with 1 mM silver nitrate solution during the first hour (Fig. 1), indicating the synthesis of AgNPs (Fig. 1). The surface plasmon resonance (SPR) peaks were found to be 437, 440, 500, and 400 nm, respectively, for AgNps from C. sinensis, I. carnea, P. major, and B.alba (Fig. 1), causing the synthesis of AgNps in the size from 2 to 100 nm (Bar et al. 2009; Philip et al. 2011, Mittal et al. 2013). Spectrophotometric absorption measurement in the wavelength ranging from 400 to 450 nm is used to characterize AgNPs (Mittal et al. 2013; Mittal et al. 2014). Swainsonine alkaloids, calystegine C1, and calystegine B2 were showed by GC-MS as chemical constituents in I. carnea leaves (De Balogh et al. 1999). Also, the leaf of P. major contained the high content of flavonoid; total phenol and tannin (Kobeasy et al. 2011 Al-Jumaily et al. 2012). Mustard had a higher antioxidant activity, moreover, quite active in stabilizing the edible oils and fatty food against rancidity and oxidative deterioration (Mariod et al. 2009). Moreover, isothiocyanates, phenolics, dithiolthiones, and dietary fiber were detected in Sinapis alba as active components (Hendrix et al. 2012). The yellow-seeded types had higher sucrose (8.7%), protein (44.5%), and oligosaccharides (2.3%). Simbaya et al and Daniel et al. confirmed that reducing silver nitrate to AgNps may be involved by polyphenols (Simbaya et al. 1995; Daniel et al. 2014). Various plant metabolites, including terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins play an important role in the bioreduction of metal ions to form nanoparticles (Makarov et al. 2014). Various functional groups of flavonoids (anthocyanins, isoflavonoids, flavonols, chalcones, flavones, and flavanones) are capable to form nanoparticles (Makarov et al. 2014; Makarov et al. 2014).
3.2 Dissipation of Fipronil and Degradation Products in Water by Silver Nanoparticles
The results of the dissipation percentage of fipronil and degradation products, fipronil amide, fipronil desulfenyl, and fipronil sulfone in the water, are presented in Fig. 2a–d. Brassica-AgNps, Ipomoea-AgNps, Camellia-AgNps, and Plantago-AgNps in water solution significantly reduced fipronil residues by 95.45% (2.95 mg/L), 90.15% (6.40 mg/L), 63.65% (32.63 mg/L), and 63.48% (23.74 mg/L) through 2 days of exposure as compared with 18.42% (53.03 mg/L) in water without AgNps (Table 1 and Fig. 2a). Fipronil dissipation in water through 2 days was arranged as Brassica-AgNps > Ipomoea-AgNps > Camellia-AgNps > Plantago-AgNps. Fipronil was decreased in the water to 98.21% (1.16 mg/L) by Brassica-AgNps, 95.93% (2.64 mg/L) by Ipomoea-AgNps, 82.44% (11.41 mg/L) by Camellia-AgNps, and 89.20% (7.02 mg/L) by Plantago-AgNps through 6 days of exposure periods as compared with 39.71% (39.19 mg/L) in the control (Fig. 2a). Fipronil amide was more greatly increased in water treated with Camellia-AgNps and Plantago-AgNps than that Ipomoea-AgNps and Brassica-AgNps (Fig. 2b). While, fipronil-desulfenyl residues was more greatly increased by Camellia-AgNps than those other treatments (Fig. 2c). On the other hand, the degradation product at retention time 1.43 min was more greatly increased by Ipomoea-AgNps than other treatments and not detected in the Plantago-AgNp treatment (Fig. 2d). Complete mineralization of chlorpyrifos was found to be 100, 140, and 180 min at 1, 2, and 3 ppm, respectively (Bootharaju and Pradeep, 2012). Similarly, the mineralization time for 1, 2, and 3 ppm malathion were found to be 100, 140, and 200 min respectively (Manimegalai et al. 2012). It is observed that the silver nanoparticles can effectively mineralize the pesticides (Manimegalai et al. 2014).It should be noted that the breakdown of fipronil in water varies according to the type of plant extract in AgNPs (Fig. 3). In green synthesis of AgNPs using plant extracts, several factors including plant source, types of organic compounds in the crude leaf extract, concentration of initial silver ions, temperature, and the type and concentration of leaf extract pigments are the key factors on the efficiency of AgNPs fabrication process (Leela and Vivekanandan 2008). Furthermore, extracts from plants may act both as reducing and stabilizing agents in nanoparticle synthesis (Makarov et al. 2014; Ghaffari-Moghaddam and Hadi-Dabanlou 2014).
Degradation of a fipronil and metabolites b fipronil desulfinyl, c fipronil amide, and d new product (NP) at retention time RT 1.43 in water by green silver nanoparticles at 2.0 and 5.0 days. Different letters represent significant differences (Tukey’s honestly significant difference test at P ≤ 0.05) among all treatments
3.3 Enhancing Phytoremediation of Fipronil-Contaminated Soil in Flooded Soil by Silver Nanoparticles
The level of fipronil, in different treatments, were measured through 6 days (Fig. 4) to determine the AgNps’ ability and P. major in removing fipronil from the soil. The contribution of Brassica- AgNps (T5), Plantago-AgNps (T4), Ipomoea-AgNps (T3), Camellia-AgNps (T6), and Plantago major (T2) alone to the degradation of fipronil in the soil were 68.8, 54.64, 43.75, 30.99, and 10.14% through 6 days, respectively. It showed that the combination of AgNps (T3-T6) played the most important role in the degradation of fipronil in the soil, followed by P. major (T2) compared with natural degradation (T1). It could be concluded that an enhancement in fipronil dissipation in phytoremediation system could possibly be achieved due to the degradation induced by the combined effects of AgNps and P. major.
The degradation of fipronil by AgNps amended with P. major in flooded soil is shown in Fig. 5. The percent removal of fipronil by Brassica-AgNps, Plantago-AgNps, Ipomoea-AgNps, Camellia-AgNps, and P. major alone in flooding soil within 6 days was 82.56, 68.41, 57.51, 44.75, and 13.76%, respectively (Fig. 5a). The degradation percentage of fipronil in the controls without AgNps and without P. major within 6 days was 3.6%. Soil moisture played an important role to increase ionization and activation of nZVI (Kim et al. 2010). This may be because soil saturation with water decreases the oxygen levels and thus prevent the oxidation of nZVI (El-Temsah and Joner, 2013). Pesticides which are persistent in aerobic environments are more readily degraded under reducing conditions (Comfort et al. 2001), thus generating a reducing environment in contaminated soils. Fipronil uptake into the P. major roots at low level and reached 3.62 mg/kg after 6 days of exposure may be due to increase log Kow (3.9–4.1). Studies of metabolism after soil incorporation carried out with [14C]fipronil on maize, sunflower, and sugar beet show an uptake of about 5% (Dieckmannet et al. 2010). Fipronil reached 2.33, 0.89, 0.0, and 0.0 mg/kg, respectively in P. major roots treated with Camellia-AgNps, Brassica-AgNps, Plantago-AgNps, and Ipomoea-AgNps, due to increasing degradation processes in soil by AgNps (Fig. 5c). Fipronil translocated into the P. major leaves and reached to less than 1.0 mg/kg in all treatments, indicating the lowing role of P. major in phytoremediation of fipronil-contaminated soil (Fig. 5e). However, the resulting fipronil amide product accumulates in large quantities in plant roots and leaves. Fipronil amide uptake into the P. major roots reached 41.82, 38.37, 15.09, 8.82, and 8.49 mg/kg by Camellia-AgNps, Ipomoea-AgNps, Plantago-AgNps, P. major, and Brassica-AgNps respectively (Fig. 5d).In the leaves, fipronil amide translocated into the P. major leaves and reached 22.08, 14.40, 13.09, 11.09, and 10.48 mg/kg by P. major, Camellia-AgNps, Ipomoea-AgNps, Brassica-AgNps, and Plantago-AgNps, respectively (Fig. 5f). Increase fipronil-amide in P. major roots and leaves, due to fipronil dissipation to the very water-soluble fipronil amide, Koc values from 96 to 203 (Gunasekara et al. 2007). Fipronil amide in soil was detected in all treatments, except in soil treated with Ipomoea-AgNps (Fig. 4b). Degradation chemicals of fipronil include fipronil desulfinyl (photodegradation product), fipronil amide (hydrolysis product and can account for up to 38% by soil degradation), fipronil sulfide (up to 17% by soil reduction), and fipronil sulfone (up to 34% by soil oxidation) (Harris 2004; Gunasekara et al. 2007). It is noted that AgNps played a major role in the breakdown of fipronil in the soil while P. major played an important role in taking the most polar break products (fipronil amide) as phytoremediation. Degradation of fipronil (R.T 2.7 min.) and fipronil amide (R.T, 1.9 min.) in soil, roots, and leaves of P. major under AgNp treatments are shown in Figs. 6 and 7.
Enhancing the degradation of fipronil in flooded soil containing Plantago major by green silver nanoparticles at 6.0 days. a Fipronil in soil. b Fipronil amid in soil. c Fipronil in roots. d Fipronil amid in roots. e A fipronil in leaves. f Fipronil amid in leaves. Different letters represent significant differences (Tukey’s honestly significant difference test at P ≤ 0.05) among all treatments
4 Conclusions
Silver nanoparticles were synthesized by environmentally safe and facile route, namely green nanotechnology. Silver nanoparticles were clearly characterized by UV-vis technique. The addition of Brassica-AgNps, Plantago-AgNps, Ipomoea-AgNps, and Camellia-AgNps, separately facilitated the degradation of fipronil in water and in flooded soil markedly. However, low uptake and translocation of fipronil by P. major roots and leaves were seen in flooded soil alone or under the influence of AgNps within 6 days of treatment. On the other hand, P. major played an important role for remediating the polar break product, fipronil amide in flooded soil alone or under the influence of AgNps within 6 days of treatment. The degradation product fipronil amide and fipronil desulfenyl were detected in water under the influence of AgNps.
References
Al-Jumaily, E.F., Hassan, A.A., & Rana, H.R. (2012). Extraction and purification of tannins from Plantago lanceolata L. and assessment their antibacterial activity on pathogenesis of enteropathogenic E. coli in vitro and in vivo. DAMA Internationa1, 2319–5037.
Alyokhin, A., Baker, M., Mota-Sanchez, D., Dively, G.,& Grafius, E. (2008). Colorado potato beetle resistance to insecticides. Am J Pot Res 85,395–413.
Bar, H., Bhui, D., Sahoo, G., Sarkar, P., Pyne, S., & Misra, A. (2009). Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 348(1), 212–216.
Bobe, A., Cooper, J. F., Coste, C. M., & Muller, M. A. (1998). Behavior of fipronil in soil under Sahelian plain field conditions. Pesticide Science, 52(3), 275–281.
Bonmatin, J. M., Marchand, P. A., Cotte, J. F., Aajoud, A., Casabianca, H., Goutailler, G., & Courtiade, M. (2007). Bees and systemic insecticides (imidacloprid, fipronil) in pollen: subnano-quantification by HPLC/MS/MS and GC/MS. In D. R. AAM, E. Capri, & T. M. Fragoulis (Eds.), Environmental fate and ecological effects of pesticide (pp. 827–824). Pavia: La Goliardica Pavese.
Bootharaju, M. S., & Pradeep, T. (2012). Understanding the degradation pathway of the pesticide, chlorpyrifos by noble metal nanoparticles. Langmuir, 28, 2671–2679.
Chandran, S. P., Minakshi, C., Renu, P., Absar, A., & Murali, S. (2006). Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnology Progress, 22, 577–583.
Chauzat, M. P., Martel, A. C., Cougoule, N., Porta, P., & Lachaize, J. (2011). An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environmental Toxicology and Chemistry, 30, 103–111.
Comfort, S. D., Shea, P. J., Machacek, T. A., Gaber, H., & Oh, B. T. (2001). Field-scale remediation of a metolachlor-contaminated spill site using zerovalent iron. Journal of Environmental Quality, 30, 1636–1643.
Daniel, S. C., Banub, B., Harshinyc, M., Nehrud, K., Ganeshb, P., Kumaranb, S., & Sivakumar, M. (2014). Ipomea carnea-based silver nanoparticle synthesis for antibacterial activity against selected human pathogens. Journal of Experimental Nanoscience, 9, 197–209.
De Balogh, K., Dimande, A. P., Lugt, J. J., Molyneux, R. J., Naude, T. W., & Welman, W. G. (1999). A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique. Journal of Veterinary Diagnostic Investigation, 11, 266–273.
Dieckmann, Y., Ishaque, M., Muenster, I., Picard, L., & Benz, A. (2010). Systemicity enhancers. Patent No. US 2010/0204045 A1. 1–21 DoW Agro Sciences (2013) DoW AgroSciences receives US EPA Registration for Sulfoxaflor.
El-Temsah, Y. S., & Joner, E. J. (2013). Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere, 92(1), 131–137.
Gan, J., Bondarenko, S., Oki, L., Haver, D., & Li, J. X. (2012). Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ Sci Technol, 46, 1489–1495.
Ghaffari-Moghaddam, M., & Hadi-Dabanlou, R. (2014). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. Journal of Industrial and Engineering Chemistry, 20(2), 739–744.
Gunasekara, A. S., Tresca, T., Kean, S., Frank, S. V., & Ronald, S. (2007). Environmental fate and toxicology of fipronil. Journal of Pesticide Science, 32(3), 189–199.
Harris, R. S.(2004). The fate of bifenthrin and Fipronil in pine bark nursery media. Louisiana State University. A Thesis. August 2004.
Hendrix, K. M., Morra, M. J., Lee, H. B., & Min, S. C. (2012). Defatted mustard seed meal-based biopolymer film development. Food Hydrocolloids, 26, 118–125.
Huang, J., Li, Q., Sun, D., Lu, Y., Su, Y., Yang, X., Wang, H., Wang, Y. (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology, 18, 10 Article ID 105104.
Huo, Y., Singh, P., Kim, Y. J., Soshnikova, V., Kang, J., Markus, J., Ahn, S., Castro-Aceituno, V., Mathiyalagan, R., Chokkalingam, M., Bae, K. S., & Yang, D. C. (2017). Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells, Nanomedicine, Biotechnol, 4, 1–13.
Kim, S. C., Yang, J. E., Ok, Y. S., Skousen, J., Kim, D. G., & Joo, J. H. (2010). Accelerated metolachlor degradation in soil by zerovalent iron and compost amendments. Bulletin of Environmental Contamination and Toxicology, 84(4), 459–464.
Kobeasy, O., Abdel-Fatah, M., Samiha, M., Abd El-Salam, Z., & El-Ola, M. M. (2011). Biochemical studies on Plantago major L. and Cyamopsis tetragonoloba L. Int J Biodvers Conserv, 3, 83–91.
Leela, A., & Vivekanandan, M. (2008). Tapping the unexploited plant resources for the synthesis of silver nanoparticles. African Journal of Biotechnology, 7(17), 3162–3165.
Lee, S. J., Mulay, P., Diebolt-Brown, B., Lackovic, M. J., Mehler, L. N., Beckman, J., Waltz, J., Prado, J. B., Mitchell, Y. A., Higgins, S. A., Schwartz, A., & Calvert, G. M. (2010). Acute illnesses associated with exposure to fipronil—surveillance data from 11 states in the United States, 2001–2007. Clinical Toxicology, 48, 737–744.
Li, S., Shen, Y., Xie, A., Xie, A., Yu, X., Qiu, L., Zhang, L., & Zhang, Q. (2007). Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chemistry, 9, 852–858.
Li M, Puyu L, Lin W, Mengyuan F, & Lijun H (2015) Determination and dissipation of fipronil and its metabolites in peanut and soil. Journal of Agricultural and Food Chemistry is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036.
Makarov, V., Love, A., Sinitsyna, O., Yaminsky, S. M., Taliansky, M., & Kalinina, N. (2014). Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae, 6(1), 35–44.
Manimegalai, G., Shanthakumar, S., & Chandan, S. (2012), Pesticide mineralization in water using silver nanoparticles incorporated on polyurethane foam. International Journal of Science and Research (IJSR).1, (3) December.
Manimegalai, G., Shanthakumar, S., & Chandan, S. (2014). Silver nanoparticles: synthesis and application in mineralization of pesticides using membrane support. Int Nano Lett, 4(105).
Mariod, A., Ibrahim, R. M., Ismail, M., & Ismail, N. (2009). Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chemistry, 116, 306–312.
Mittal, A. K., Chisti, Y., & Banerjee, U. C. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnology Advances, 31(2), 346–356.
Mittal, A. K., Bhaumik, J., Kumar, S., & Banerjee, U. C. (2014). Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. Journal of Colloid and Interface Science, 415, 39–47.
Ndikau, M., Naumih, M. N., Dickson, M. A., & Eric, M. (2017). Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. International Journal of Analytical Chemistry, 2017(8108504), 9. https://doi.org/10.1155/2017/8108504.
Ohi, M., Dalsente, R. P. R., Andrade, A. J. M., & Nascimento, A. J. (2004). Reproductive adverse effects of fipronil in Wistar rats. Toxicology Letters, 146(2), 121–127.
Okumura, F., Raquel, B., Ednilsom, O., Albérico, B., & Luiz, H. (2016). Electrochemical and quantum chemical investigations of the insecticide fipronil. Journal of the Brazilian Chemical Society, 27, 925–932.
Pei, Z., Yitong, L., Baofeng, L., & Gan, J. J. (2004). Dynamics of fipronil residue in vegetable field ecosystem. Chemosphere, 57, 1691–1696.
Philip, D., Unni, C., Aromal, S. A., & Vidhu, V. (2011). Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 78(2), 899–904.
Raquel, P., Tercariol, G., & Godinho, A. F. (2011). Behavioral effects of acute exposure to the insecticide fipronil. Pesticide Biochemistry and Physiology, 99, 221–225.
Raveton, M., Aajoud, A., Willison, J., Cherifi, M., Tissut, M., & Ravanel, P. (2007). Soil distribution of fipronil and its metabolites originating from a seed-coated formulation. Chemosphere, 69(7), 1124–1129.
Shuai, X., Jingyu, C., & Chittaranjan, R. (2011). Adsorption, transport and degradation of fipronil termiticide in three Hawaii soils. Pest Management Science, 68, 731–739.
Simbaya, J., Slominski, B. A., Rakow, G., Campbell, L. D., Keith, R., Downey, R., & Bell, J. M. (1995). Quality characteristics of yellow-seeded Bmmica seed meals: protein, carbohydrates and dietary fibre components. Journal Science Food Agriculture Chemistry, 43, 2062–2066.
Subha, V., Kirubanandan, S., & Renganathan, S. (2016). Green synthesis of silver nanoparticles from a novel medicinal plant source roots extract of Mukia maderaspatana. Colloid and Surface Science, 1(1), 14–17.
US Environmental Protection Agency (1996). Partition coefficient (N-octanol/water) estimation by liquid chromatography: U.S. EPA Office of Prevention, Pesticides, and Toxic Substances, accessed October 15, 2002, at URL http://www.epa.gov/docs/OPPTS_Harmonized/830_Product_Properties_Test_Guidelines/ Series/830–7570.pdf.
Vilchis-Nestor, A. R., Sánchez-Mendieta, V. S., Camacho-L’opez, M. A., G’omez-Espinosa, R.M., Camacho-L’opez, M. A., & Arenas-Alatorre, J.A. (2008). Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract, Materials Letters, 62, 17-183103–3105.
Von White, G., Kerscher, P., Brown, R. M., Morella, J. D., McAllister, W., Dean, D., & Kitchens, C. L. (2012). Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. Journal of Nanomaterials, 2012(730746), 12.
Wang, T., Jin, X., Chen, Z., Megharaj, M., & Naidu, R. (2014). Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ, 466, 210–213.
Acknowledgements
The author is most grateful to Professor Dr. Mustafa Abdel-Rahim, director of the Central Laboratory for soil, food, and feedstuffs ISO-17025, and the Faculty of Development and Technology, Zagazig University, Zagazig, Egypt, for their collaboration in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romeh, A.A.A. Green Silver Nanoparticles for Enhancing the Phytoremediation of Soil and Water Contaminated by Fipronil and Degradation Products. Water Air Soil Pollut 229, 147 (2018). https://doi.org/10.1007/s11270-018-3792-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3792-3