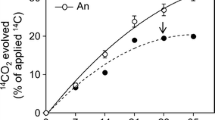
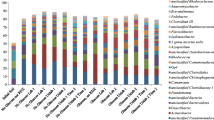
Abstract
The aim of this work was to investigate the susceptibility of the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) to biodegradation in a range of soils and to identify RDX-degrading organisms using stable isotope probing (SIP). RDX degradation was examined in ten soils, primarily from agricultural areas. RDX biodegradation was observed in six samples and only when the microcosms were not aerated. For one soil, 15N- and 13C-based DNA SIP was used to identify the microorganisms responsible for RDX degradation. Two RDX concentrations were examined (10 and 20 mg/L), however, only the higher concentration resulted in a significant SIP signal. In these ultracentrifugation fractions, one terminal restriction fragment length polymorphism (TRFLP) fragment (260 bp) showed a reliable trend of label uptake. This fragment was of higher relative abundance in the heavier fractions from labeled samples compared with the heavier fractions from the unlabeled control samples, indicating that the organism producing this fragment was responsible for label uptake (hence RDX degradation). Partial 16S rRNA gene sequencing indicated the organisms represented by fragment 260 bp belonged to either Sphingobacteria (phylum Bacteroidetes) or the phylum Acidobacteria. To date, these organisms have not previously been directly linked to RDX degradation. The 16S rRNA sequences were compared with the NCBI database and, in all cases, were most similar to uncultured bacteria. The results suggest SIP is a viable method for discovering novel, previously uncultured, RDX degraders.





Similar content being viewed by others
References
Adrian, N. R., & Arnett, C. M. (2004). Anaerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Acetobacterium malicum strain HAAP-1 isolated from a methanogenic mixed culture. Current Microbiology, 48(5), 332–340.
Adrian, N. R., & Chow, T. (2001). Identification of hydroxylamino-dinitroso-1,3,5-triazine as a transient intermediate formed during the anaerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Environmental Toxicology and Chemistry, 20(9), 1874–1877.
Annamaria, H., Manno, D., et al. (2010). Biodegradation of RDX and MNX with Rhodococcus sp. strain DN22: new insights into the degradation pathway. Environmental Science & Technology, 44(24), 9330–9336.
Arnett, C. M., & Adrian, N. R. (2009). Cosubstrate independent mineralization of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a Desulfovibrio species under anaerobic conditions. Biodegradation, 20(1), 15–26.
Arnett, C. M., Adrian, N. R., et al. (2009). Sulfate-mediated bacterial population shift in a hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX)-degrading anaerobic enrichment culture. Bioremediation Journal, 13(1), 52–63.
Barns, S. M., Cain, E. C., et al. (2007). Acidobactetia phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Applied and Environmental Microbiology, 73(9), 3113–3116.
Beller, H. R. (2002). Anaerobic biotransformation of RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) by aquifer bacteria using hydrogen as the sole electron donor. Water Research, 36(10), 2533–2540.
Bhushan, B., Halasz, A., et al. (2004). Chemotaxis-mediated biodegradation of cyclic nitramine explosives RDX, HMX, and CL-20 by Clostridium sp. EDB2. Biochemical Biophysical Research Communications, 316, 816–821.
Bhushan, B., Trott, S., et al. (2003). Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a rabbit liver cytochrome p450: insight into the mechanism of RDX biodegradation by Rhodococcus sp strain DN22. Applied and Environmental Microbiology, 69(3), 1347–1351.
Binks, P. R., Nicklin, S., et al. (1995). Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas-maltophilia Pb1. Applied and Environmental Microbiology, 61(4), 1318–1322.
Boopathy, R., Gurgas, M., et al. (1998). Metabolism of explosive compounds by sulfate-reducing bacteria. Current Microbiology, 37(2), 127–131.
Branco, R., Chung, A. P., et al. (2005). Impact of chromium-contaminated wastewaters on the microbial community of a river. FEMS Microbiology Ecology, 54(1), 35–46.
Chan, O. C., Yang, X. D., et al. (2006). 16S rRNA gene analyses of bacterial community structures in the soils of evergreen broad-leaved forests in south-west China. Fems Microbiology Ecology, 58(2), 247–259.
Cho, K. C., Lee, D. G., et al. (2013). Application of 13C-stable isotope probing to identify RDX-degrading microorganisms in groundwater. Environmental Pollution, 178, 350–360.
Clement, B. G., Kehl, L. E., et al. (1998). Terminal restriction fragment patterns (TRFPs), a rapid, PCR-based method for the comparison of complex bacterial communities. Journal of Microbiological Methods, 31(3), 135–142.
Coates, J. D., Ellis, D. J., et al. (1999). Geothrix fermentans gen. nov., sp nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. International Journal of Systematic Bacteriology, 49, 1615–1622.
Cole, J. R., Wang, Q., et al. (2009). The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Research, 37, D141–D145.
Coleman, N. V., Nelson, D. R., et al. (1998). Aerobic biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biology & Biochemistry, 30(8–9), 1159–1167.
Coleman, N. V., Spain, J. C., et al. (2002). Evidence that RDX biodegradation by Rhodococcus strain DN22 is plasmid-borne and involves a cytochrome p-450. Journal of Applied Microbiology, 93(3), 463–472.
Crocetti, G. R., Banfield, J. F., et al. (2002). Glycogen-accumulating organisms in laboratory-scale and full-scale wastewater treatment processes. Microbiology, 148, 3353–3364.
Cupples, A. M., Shaffer, E. A., et al. (2007). DNA buoyant density shifts during N-15-DNA stable isotope probing. Microbiological Research, 162(4), 328–334.
Cupples, A. M., & Sims, G. K. (2007). Identification of in situ 2,4-dichlorophenoxyacetic acid-degrading soil microorganisms using DNA-stable isotope probing. Soil Biology & Biochemistry, 39(1), 232–238.
Dunbar, J., Takala, S., et al. (1999). Levels of bacterial community diversity in four arid soils compared by cultivation and 16S rRNA gene cloning. Applied and Environmental Microbiology, 65(4), 1662–1669.
Eaton, H. L., De Lorme, M., et al. (2011). Ovine ruminal microbes are capable of biotransforming hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Microbial Ecology, 62(2), 274–286.
Eichorst, S. A., Breznak, J. A., et al. (2007). Isolation and characterization of soil bacteria that define Teniglobus gen. nov., in the phylum Acidobacteria. Applied and Environmental Microbiology, 73(8), 2708–2717.
Fournier, D., Halasz, A., et al. (2002). Determination of key metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine with Rhodococcus sp strain DN22. Applied and Environmental Microbiology, 68(1), 166–172.
Fukunaga, Y., Kurahashi, M., et al. (2008). Acanthopleuribacter pedis gen. nov., sp nov., a marine bacterium isolated from a chiton, and description of Acanthopleuribacteraceae fam.nov., Acanthopleuribacterales ord. nov., Holophagaceae fam. nov., Holophagales ord. nov and Holophagae classis nov in the phylum 'Acidobacteria'. International Journal of Systematic and Evolutionary Microbiology, 58, 2597–2601.
Fuller, M. E., McClay, K., et al. (2010a). Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) bioremediation in groundwater: are known RDX-degrading bacteria the dominant players? Bioremediation Journal, 14(3), 121–134.
Fuller, M. E., Perreault, N., et al. (2010b). Microaerophilic degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by three Rhodococcus strains. Letters in Applied Microbiology, 51(3), 313–318.
Gregory, K. B., Larese-Casanova, P., et al. (2004). Abiotic transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine by fell bound to magnetite. Environmental Science & Technology, 38(5), 1408–1414.
Hawari, J., Beaudet, S., et al. (2000a). Microbial degradation of explosives: biotransformation versus mineralization. Applied Microbiology and Biotechnology, 54(5), 605–618.
Hawari, J., Halasz, A., et al. (2000b). Characterization of metabolites during biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) with municipal anaerobic sludge. Applied and Environmental Microbiology, 66(6), 2652–2657.
Hugenholtz, P., Goebel, B. M., et al. (1998). Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity (vol 180, pg 4765, 1998). Journal of Bacteriology, 180(24), 6793–6793.
Indest, K. J., Crocker, F. H., et al. (2007). A TaqMan polymerase chain reaction method for monitoring RDX-degrading bacteria based on the xplA functional gene. Journal of Microbiological Methods, 68(2), 267–274.
Indest, K. J., Jung, C. M., et al. (2010). Functional characterization of pGKT2, a 182-kilobase plasmid containing the xplAB genes, which are involved in the degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine by Gordonia sp. strain KTR9. Applied and Environmental Microbiology, 76(19), 6329–6337.
Janssen, P. H. (2006). Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Applied and Environmental Microbiology, 72(3), 1719–1728.
Khan, M. I., Lee, J., et al. (2012). Microbial degradation and toxicity of hexahydro-1,3,5-trinitro-1,3,5-triazine. Journal of Microbiology and Biotechnology, 22(10), 1311–1323.
Kishimoto, N., Kosako, Y., et al. (1991). Acidobacterium capsulatum gen. nov., sp. nov.: an acidophilic chemoorganotrophic bacterium containing menaquinone from acidic mineral environment. Current Microbiology, 22(1), 1–7.
Kitts, C. L., Cunningham, D. P., et al. (1994). Isolation of 3 hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitramine explosive-contaminated soil. Applied and Environmental Microbiology, 60(12), 4608–4611.
Kitts, C. L., Green, C. E., et al. (2000). Type I nitroreductases in soil enterobacteria reduce TNT (2,4,6-trinitrotoluene) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine). Canadian Journal of Microbiology, 46(3), 278–282.
Kleinsteuber, S., Muller, F. D., et al. (2008). Diversity and in situ quantification of Acidobacteria subdivision 1 in an acidic mining lake. Fems Microbiology Ecology, 63(1), 107–117.
Koch, I. H., Gich, F., et al. (2008). Edaphobacter modestus gen. nov., sp nov., and Edaphobacter aggregans sp nov., acidobacteria isolated from alpine and forest soils. International Journal of Systematic and Evolutionary Microbiology, 58, 1114–1122.
Kwon, M. J., & Finneran, K. T. (2008a). Biotransformation products and mineralization potential for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in abiotic versus biological degradation pathways with anthraquinone-2,6-disulfonate (AQDS) and Geobacter metallireducens. Biodegradation, 19(5), 705–715.
Kwon, M. J., & Finneran, K. T. (2008b). Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) biodegradation kinetics amongst several Fe(III)-reducing genera. Soil & Sediment Contamination, 17(2), 189–203.
Kwon, M. J., & Finneran, K. T. (2010). Electron shuttle-stimulated RDX mineralization and biological production of 4-nitro-2,4-diazabutanal (NDAB) in RDX-contaminated aquifer material. Biodegradation, 21(6), 923–937.
Kwon, M. J., O'Loughlin, E. J., et al. (2011). Geochemical and microbiological processes contributing to the transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in contaminated aquifer material. Chemosphere, 84(9), 1223–1230.
Larese-Casanova, P., & Scherer, M. M. (2008). Abiotic transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by green rusts. Environmental Science & Technology, 42(11), 3975–3981.
Lee, S. H., Ka, J. O., et al. (2008). Members of the phylum Acidobacteria are dominant and metabolically active in rhizosphere soil. FEMS Microbiology Letters, 285(2), 263–269.
Liesack, W., Bak, F., et al. (1994). Holophaga foetida gen-nov, sp-nov, a new, homoacetogenic bacterium degrading methoxylated aromatic compounds. Archives of Microbiology, 162(1–2), 85–90.
Liu, W. T., Marsh, T. L., et al. (1997). Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Applied and Environmental Microbiology, 63(11), 4516–4522.
Livermore, J., Jin, Y. O., et al. (2013). Microbial community dynamics during acetate biostimulation of RDX-contaminated groundwater. Environmental Science & Technology, 47(14), 7672–7678.
Ludwig, W., Bauer, S. H., et al. (1997). Detection and in situ identification of representatives of a widely distributed new bacterial phylum. Fems Microbiology Letters, 153(1), 181–190.
Luo, C. L., Xie, S. G., et al. (2009). Identification of a novel toluene-degrading bacterium from the candidate phylum TM7, as determined by DNA stable isotope probing. Applied and Environmental Microbiology, 75(13), 4644–4647.
Luo, W., D'Angelo, E. M., et al. (2008). Organic carbon effects on aerobic polychlorinated biphenyl removal and bacterial community composition in soils and sediments. Chemosphere, 70(3), 364–373.
Macbeth, T. W., Cummings, D. E., et al. (2004). Molecular characterization of a dechlorinating community resulting from in situ biostimulation in a trichloroethene-contaminated deep, fractured basalt aquifer and comparison to a derivative laboratory culture. Applied and Environmental Microbiology, 70(12), 7329–7341.
McCormick, N. G., Cornell, J. H., et al. (1981). Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine. Applied and Environmental Microbiology, 42(5), 817–823.
Meisinger, D. B., Zimmermann, J., et al. (2007). In situ detection of novel Acidobacteria in microbial mats from a chemolithoautotrophically based cave ecosystem (Lower Kane Cave, WY, USA). Environmental Microbiology, 9(6), 1523–1534.
Mendez, M. O., Neilson, J. W., et al. (2008). Characterization of a bacterial community in an abandoned semiarid lead-zinc mine tailing site. Applied and Environmental Microbiology, 74(12), 3899–3907.
Moshe, S. S. B., Ronen, Z., et al. (2009). Sequential biodegradation of TNT, RDX and HMX in a mixture. Environmental Pollution, 157(8–9), 2231–2238.
Naja, G., Halasz, A., et al. (2008). Degradation of hexahydro-1,3,5-trinitro-1,15-triazine (RDX) using zerovalent iron nanoparticles. Environmental Science & Technology, 42(12), 4364–4370.
Oh, S. Y., Chiu, P. C., et al. (2008). Reductive transformation of 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and nitroglycerin by pyrite and magnetite. Journal of Hazardous Materials, 158(2–3), 652–655.
Perumbakkam, S., & Craig, A. M. (2012). Biochemical and microbial analysis of ovine rumen fluid incubated with 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX). Current Microbiology, 65(2), 195–201.
Pudge, I. B., Daugulis, A. J., et al. (2003). The use of Enterobacter cloacae ATCC 43560 in the development of a two-phase partitioning bioreactor for the destruction of hexahydro-1,3,5-trinitro-1,3,5-s-triazine (RDX). Journal of Biotechnology, 100(1), 65–75.
Quaiser, A., Lopez-Garcia, P., et al. (2008). Comparative analysis of genome fragments of Acidobacteria from deep Mediterranean plankton. Environmental Microbiology, 10(10), 2704–2717.
Regan, K. M., & Crawford, R. L. (1994). Characterization of Clostridium bifermentans and its biotransformation of 2,4,6-trinitrotoluene (TNT) and 1,3,5-triaza-1,3,5-trinitrocyclohexane (RDX). Biotechnology Letters, 16(10), 1081–1086.
Ringelberg, D., Richmond, M., et al. (2008). Utility of lipid biomarkers in support of bioremediation efforts at army sites. Journal of Microbiological Methods, 74(1), 17–25.
Roh, H., Yu, C. P., et al. (2009). Identification of hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading microorganisms via 15N-stable isotope probing. Environmental Science & Technology, 43(7), 2505–2511.
Ronen, Z., Yanovich, Y., et al. (2008). Metabolism of the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in a contaminated vadose zone. Chemosphere, 73(9), 1492–1498.
Rylott, E. L., Lorenz, A., et al. (2011). Biodegradation and biotransformation of explosives. Current Opinion in Biotechnology, 22(3), 434–440.
Sahu, A. K., Conneely, T., et al. (2009). Hydrogenotrophic denitrification and perchlorate reduction in ion exchange brines using membrane biofilm reactors. Biotechnology and Bioengineering, 104(3), 483–491.
Seth-Smith, H. M. B., Edwards, J., et al. (2008). The explosive-degrading cytochrome P450 system is highly conserved among strains of Rhodococcus spp. Applied and Environmental Microbiology, 74(14), 4550–4552.
Seth-Smith, H. M. B., Rosser, S. J., et al. (2002). Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Applied and Environmental Microbiology, 68(10), 4764–4771.
Sherburne, L. A., Shrout, J. D., et al. (2005). Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) degradation by Acetobacterium paludosum. Biodegradation, 16(6), 539–547.
Singh, B., Kaur, J., et al. (2012). Microbial remediation of explosive waste. Critical Reviews in Microbiology, 38(2), 152–167.
Singh, J., Comfort, S. D., et al. (1998). Remediating RDX-contaminated water and soil using zero-valent iron. Journal of Environmental Quality, 27(5), 1240–1245.
Singh, R., Soni, P., et al. (2009). Biodegradation of high explosive production effluent containing RDX and HMX by denitrifying bacteria. World Journal of Microbiology & Biotechnology, 25(2), 269–275.
Sun, W. M., & Cupples, A. M. (2012). Diversity of five anaerobic toluene-degrading microbial communities investigated using stable isotope probing. Applied and Environmental Microbiology, 78(4), 972–980.
Sun, W. M., Sun, X. X., et al. (2012). Anaerobic methyl tert-butyl ether-degrading microorganisms identified in wastewater treatment plant samples by stable isotope probing. Applied and Environmental Microbiology, 78(8), 2973–2980.
Sun, W. M., Xie, S. G., et al. (2010). Direct link between toluene degradation in contaminated-site microcosms and a Polaromonas strain. Applied and Environmental Microbiology, 76(3), 956–959.
Thompson, K. T., Crocker, F. H., et al. (2005). Mineralization of the cyclic nitramine explosive hexahydro-1,3,5-trinitro-1,3,5-triazine by Gordonia and Williamsia spp. Applied and Environmental Microbiology, 71(12), 8265–8272.
Van Aken, B., Yoon, J. M., et al. (2004). Biodegradation of nitro-substituted explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, an octahydro-1,3,5,7-tetranitro-1,3,5-tetrazocine by a phytosymbiotic Methylobacterium sp associated with poplar tissues (Populus deltoides x nigra DN34). Applied and Environmental Microbiology, 70(1), 508–517.
Xie, S. G., Sun, W. M., et al. (2010). Stable isotope probing identifies novel m-xylene degraders in soil microcosms from contaminated and uncontaminated sites. Water Air and Soil Pollution, 212(1–4), 113–122.
Xie, S. G., Sun, W. M., et al. (2011). Novel aerobic benzene degrading microorganisms identified in three soils by stable isotope probing. Biodegradation, 22(1), 71–81.
Ye, J., Singh, A., et al. (2004). Biodegradation of nitroaromatics and other nitrogen-containing xenobiotics. World Journal of Microbiology & Biotechnology, 20(2), 117–135.
Young, D. M., Unkefer, P. J., et al. (1997). Biotransformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by a prospective consortium and its most effective isolate Serratia marcescens. Biotechnology and Bioengineering, 53(5), 515–522.
Zhang, C. L., & Hughes, J. B. (2003). Biodegradation pathways of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Clostridium acetobutylicum cell-free extract. Chemosphere, 50(5), 665–671.
Zhang, L., & Xu, Z. H. (2008). Assessing bacterial diversity in soil. Journal of Soils and Sediments, 8(6), 379–388.
Zhao, J. S., Greer, C. W., et al. (2004a). Biodegradation of the nitramine explosives hexahydro-1,3,5-trinitro-1,3,5-triazine and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in cold marine sediment under anaerobic and oligotrophic conditions. Canadian Journal of Microbiology, 50(2), 91–96.
Zhao, J. S., Halasz, A., et al. (2002). Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine and its mononitroso derivative hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine by Klebsiella pneumoniae strain SCZ-1 isolated from an anaerobic sludge. Applied and Environmental Microbiology, 68(11), 5336–5341.
Zhao, J. S., Manno, D., et al. (2005). Shewanella sediminis sp nov., a novel Na+-requiring and hexahydro-1,3,5-trinitro-1,3,5-trinitro-degrading bacterium from marine sediment. International Journal of Systematic and Evolutionary Microbiology, 55, 1511–1520.
Zhao, J. S., Manno, D., et al. (2006). Shewanella halifaxensis sp nov., a novel obligately respiratory and denitrifying psychrophile. International Journal of Systematic and Evolutionary Microbiology, 56, 205–212.
Zhao, J. S., Paquet, L., et al. (2003). Metabolism of hexahydro-1,3,5-trinitro-1,3,5-triazine through initial reduction to hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine followed by denitration in Clostridium bifermentans HAW-1. Applied Microbiology and Biotechnology, 63(2), 187–193.
Zhao, J. S., Paquet, L., et al. (2004b). Metabolism of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine by Clostridium bifermentans strain HAW-1 and several other H2 producing fermentative anaerobic bacteria. FEMS Microbiology Letters, 237(1), 65–72.
Zhao, J. S., Spain, J., et al. (2004c). Phylogeny of cyclic nitramine-degrading psychrophilic bacteria in marine sediment and their potential role in the natural attenuation of explosives. FEMS Microbiology Ecology, 49(3), 349–357.
Acknowledgments
This project was supported by the Strategic Environmental Research and Development Program. The following people are acknowledged for their work on this project. Thanks to Dan Williams for sample collection and Yanglang Pan for analytical assistance. The authors thank Stuart Strand and Peter Andeer for supplying R. rhodochrous 11Y genomic DNA for the xplA studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 13 kb)
Rights and permissions
About this article
Cite this article
Jayamani, I., Manzella, M.P. & Cupples, A.M. RDX Degradation Potential in Soils Previously Unexposed to RDX and the Identification of RDX-Degrading Species in One Agricultural Soil Using Stable Isotope Probing. Water Air Soil Pollut 224, 1745 (2013). https://doi.org/10.1007/s11270-013-1745-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1745-4