Abstract
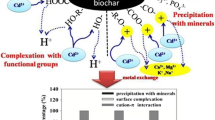
Biochar has great potential as a soil amendment to immobilize heavy metals, thereby reducing their bioavailability. In this study, biochars derived from chicken manure and green waste were compared with commercial activated carbon (AC) and laboratory produced black carbon (BC) for the sorption of Pb and Cd. Sorption kinetics and equilibrium sorption isotherms for Pb and Cd were obtained for the char materials and the data were fitted to kinetic and sorption isotherm models.. Chicken manure-derived biochar (CM) showed the highest sorption capacity for both Pb and Cd, and the Pb sorption by biochars was higher than the Cd sorption because of the precipitation of Pb with various ions released from the biochars such as carbonate, phosphate, and sulfate. The sorption data for both Pb and Cd were better represented by the pseudo-second order kinetic model than the pseudo-first order kinetic model, which indicates chemical sorption between biochar and metals. For the isotherm studies, char materials was mixed with various amount of Pb or Cd solutions and the remaining metal concentration was measured. The equilibrium sorption data followed a Langmuir isotherm with a maximum sorption capacity of 6.8–11 and 1.7–8.0 mg/g by biochars for Pb and Cd, respectively. Furthermore, CM immobilized Pb and Cd up to 93.5 and 88.4 %, respectively, while BC was not effective in the immobilization of Pb in soil. Overall, the sorption experiments in solution and the immobilization experiment in soil showed that biochars are more effective than AC in the sorption of Pb and Cd, and that they have the potential to be used as a soil amendment to remediate metal-contaminated soil.







Similar content being viewed by others
References
Adib, F., Bagreev, A., & Bandosz, T. J. (1999). Effect of surface characteristics of wood-based activated carbons on adsorption of hydrogen sulfide. Journal of Colloid and Interface Science, 214, 407-415.
Baldock, J. A., & Smernik, R. J. (2002). Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Organic Geochemistry, 33, 1093–1109.
Beesley, L., & Marmiroli, M. (2011). The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environmental Pollution, 159, 474–480.
Beesley, L., Moreno-Jiménez, E., & Gomez-Eyles, J. L. (2010). Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environmental Pollution, 158, 2282–2287.
Boehm, H. (1994). Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon, 32, 759–769.
Cao, X., & Harris, W. (2010). Properties of dairy–manure-derived biochar pertinent to its potential use in remediation. Bioresource Technology, 101, 5222–5228.
Cao, X., Ma, L., Gao, B., & Harris, W. (2009). Dairy–manure derived biochar effectively sorbs lead and atrazine. Environmental Science & Technology, 43, 3285–3291.
Chen, X., Chen, G., Chen, L., Chen, Y., Lehmann, J., McBride, M. B., & Hay, A. G. (2011). Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresource Technology, 101, 8877–8884.
Chen, B., Zhou, D., & Zhu, L. (2008). Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environmental Science & Technology, 42, 5137–5143.
Choppala, G., Bolan, N., Megharaj, M., Chen, Z., & Naidu, R. (2012). The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. Journal of Environmental Quality, 41, 1175–1184.
Chun, Y., Sheng, G., Chiou, C. T., & Xing, B. (2004). Compositions and sorption properties of crop residue-derived chars. Environmental Science & Technology, 38, 4649–4655.
Gaunt, J. L., & Lehmann, J. (2008). Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environmental Science & Technology, 42, 4152–4158.
Hartley, W., Dickinson, N. M., Riby, P., & Lepp, N. W. (2009). Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environmental Pollution, 157, 2654–2662.
Harvey, O. R., Herbert, B. E., Rhue, R. D., & Kuo, L. J. (2011). Metal interactions at the biochar–water interface: energetics and structure–sorption relationships elucidated by flow adsorption microcalorimetry. Environmental Science & Technology, 45, 5550–5556.
Ho, Y. S. (2006). Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Research, 40, 119–125.
Ho, Y. S., Huang, C. T., & Huang, H. W. (2002). Equilibrium sorption isotherm for metal ions on tree fern. Process Biochemistry, 37, 1421–1430.
Ho, Y. S., Ng, J. C. Y., & McKay, G. (2000). Kinetics of pollutant sorption by biosorbents: review. Separation & Purification Reviews, 29, 189–232.
Inyang, M., Gao, B., Ding, W., Pullammanappallil, P., Zimmerman, A. R., & Cao, X. (2011). Enhanced lead sorption by biochar derived from anaerobically digested sugarcane bagasse. Separation Science and Technology, 46, 1950–1956.
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A. R., Pullammanappallil, P., & Cao, X. (2012). Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresource Technology, 110, 50–56.
Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182.
Keiluweit, M., Nico, P. S., Johnson, M. G., & Kleber, M. (2010). Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environmental Science & Technology, 44, 1247–1253.
Li, Y., Wang, S., Wei, J., Zhang, X., Xu, C., & Luan, Z. (2002). Lead adsorption on carbon nanotubes. Chemical Physics Letters, 357, 263–266.
Liu, Z., & Zhang, F. S. (2009). Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. Journal of Hazardous Materials, 167, 933–939.
Mohan, D., Pittman, C. U., Bricka, M., Smith, F., Yancey, B., Mohammad, J., Steele, P. H., Alexandre-Franco, M. F., Gómez-Serrano, V., & Gong, H. (2007). Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. Journal of Colloid and Interface Science, 310, 57–73.
Moreno–Castilla, C., Lopez–Ramon, M., & Carrasco–Marin, F. (2000). Changes in surface chemistry of activated carbons by wet oxidation. Carbon, 38, 1995–2001.
NEPC, National Environment Protection Council. (1999). National Environment Protection (Assessment of Site Contamination) Measure: Schedule B(1) Guideline on the Investigation Levels for Soil and Groundwater, Australia
Park, J. H., Bolan, N., Megharaj, M., & Naidu, R. (2011a). Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). Journal of Environmental Management, 92, 1115–1120.
Park, J. H., Choppala, G. K., Bolan, N. S., Chung, J. W., & Chuasavathi, T. (2011b). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant and Soil, 348, 439–451.
Pellera, F. M., Giannis, A., Kalderis, D., Anastasiadou, K., Stegmann, R., Wang, J. Y., & Gidarakos, E. (2012). Adsorption of Cu (II) ions from aqueous solutions on biochars prepared from agricultural by-products. Journal of Environmental Management, 96, 35–42.
Ragauskas, A. J., Williams, C. K., Davison, B. H., Britovsek, G., Cairney, J., Eckert, C. A., Frederick, W. J., Jr., Hallett, J. P., Leak, D. J., & Liotta, C. L. (2006). The path forward for biofuels and biomaterials. Science, 311, 484–489.
Rangel–Mendez, J., & Streat, M. (2002). Adsorption of cadmium by activated carbon cloth: influence of surface oxidation and solution pH. Water Research, 36, 1244–1252.
Rudzinski, W., & Plazinski, W. (2006). Kinetics of solute adsorption at solid/solution interfaces: a theoretical development of the empirical pseudo-first and pseudo-second order kinetic rate equations, based on applying the statistical rate theory of interfacial transport. Journal of Physical Chemistry, 110, 16514-16525.
Tan, T. C., & Teo, T. C. (1987). Combined effect of carbon dosage and initial adsorbate concentration on the adsorption isotherm of heavy metals on activated carbon. Water Research, 21, 1183–1188.
Tomaszewski, J. E., Werner, D., & Luthy, R. G. (2007). Activated carbon amendment as a treatment for residual DDT in sediment from a superfund site in San Francisco Bay, Richmond, California, USA. Environmental Toxicology and Chemistry, 26, 2143–2150.
Tong, X., Li, J., Yuan, J., & Xu, R. (2011). Adsorption of Cu (II) by biochars generated from three crop straws. Chemical Engineering Journal, 172, 828–834.
Uchimiya, M., Lima, I. M., Thomas Klasson, K., Chang, S. C., Wartelle, L. H., & Rodgers, J. E. (2010). Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. Journal of Agricultural and Food Chemistry, 58, 5538–5544.
Uchimiya, M., Klasson, K. T., Wartelle, L. H., & Lima, I. M. (2011). Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere, 82, 1431–1437.
Van Zwieten, L. V., Kimber, S., Morris, S., Chan, K. Y., Downie, A., Rust, J., Joseph, S., & Cowie, A. (2009). Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant and Soil, 327, 235-246.
Wang, X. S., Miao, H. H., He, W., & Shen, H. L. (2011). Competitive adsorption of Pb (II), Cu (II), and Cd (II) ions on wheat-residue derived black carbon. Journal of Chemical & Engineering Data, 56, 444–449.
Wong, Y. C., Szeto, Y. S., Cheung, W. H., & McKay, G. (2004). Adsorption of acid dyes on chitosan-equilibrium isotherm analyses. Process Biochemistry, 39, 695-704.
Acknowledgments
This study was supported by Korea Ministry of Environment as “The GAIA Project (G112-17003-0043-0) and Gyeongnam National University of Science and Technology Grant. The authors thank Byoung Hwan Seo and Si Young Choi for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: R Naidu, Euan Smith, MH Wong, Megharaj Mallavarapu, Nanthi Bolan, Albert Juhasz, and Enzo Lombi
This article is part of the Topical Collection on Remediation of Site Contamination
Rights and permissions
About this article
Cite this article
Park, J.H., Choppala, G., Lee, S.J. et al. Comparative Sorption of Pb and Cd by Biochars and Its Implication for Metal Immobilization in Soils. Water Air Soil Pollut 224, 1711 (2013). https://doi.org/10.1007/s11270-013-1711-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1711-1