Abstract
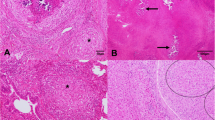
To date, the public health relevance of mycobacterial infections in pigs is not well investigated despite high risk of infection. Recently, there has been a documented increase in opportunistic infections and risk of acquiring opportunistic mycobacterial infections in HIV/AIDS patients in Mubende district; unfortunately, there has been no published information on the epidemiology of mycobacterial infections in this area. This study was carried out between September 2008 and February 2009. Investigations were done to assess the prevalence and associated risk factors of mycobacterial infections in slaughtered pigs in Mubende district of Uganda. A total of 997 pigs (53.7% male and 46.3% female) from 31 different slaughterhouses were examined for the presence of lesions compatible with TB and mycobacterial infections. Pathologic tissue specimens were collected for culturing and isolation of mycobacteria. A cross-sectional technique was used based on convenient visits to slaughterhouses but random selection of individual slaughtered pigs for a detailed post-mortem inspection on a daily basis. The results reflected a 9.3% and 3.1% (95% CI) prevalence of Mycobacterium species based on necropsy examinations and culture isolation, respectively. The highest prevalence of mycobacterial infection was recorded in Buwekula County (the mixed agro-zone) whilst the lowest was in Kassanda County (pastoral zone). A multivariable logistical regression analysis identified age (P ≤ 0.001) and sex (P ≤ 0.05) as risk factors for mycobacterial infections in pigs. Post-estimation statistics of the regression model evaluation and validation fit it well into the data (HL, χ 2 = 5.9; P = 0.69 for necropsy, HL χ 2 = 2.9; P = 0.94 for culturing). This study documented a high prevalence of mycobacterial infections in slaughter pigs in Mubende district. The fact that pigs and human often share common housing and environment poses a high risk of zoonotic transmission. This then warrants further molecular investigation to identify the specific Mycobacterium species and their public health importance in this area.


Similar content being viewed by others
References
Anonymous, 2004a. Bankable investment project profile: Livestock Development Project, Food and Agricultural Organisation and New Partnership for Africa’s Development report (III of VI), 1–25
Anonymous, 2004b. District state of environment report Mubende, National Environment Management Authority, 1–129
Anonymous, 2005. First precise maps of Ugandan poverty published (book pinpoints poorest people at the county level), Uganda Bureau of Statistics 1–4
Anonymous, 2007. Uganda Districts: Information Handbook, Fountain Publishers, 139–149
Anonymous, 2009. Uganda: Tuberculosis profile, United States Agency for International Development report, http://www1.usaid.gov/our_work/global_health/id/tuberculosis/countries/africa/uganda.pdf
Awa, D.N., Njoya, A.C., Tama, N. and Ekue, F.N., 1999. Diseases in north Cameroon: The health status of pigs in north Cameroon, http://pigtrop.cirad.fr/subjects/animal_health/diseases_in_north_cameroon
Brandt, L., Cunha, J.F., Weinreich, A., Chilima, B., Hirsch, P., Appelberg, R. and Andersen, P., 2002. Failure of the Mycobacterium bovis BCG vaccine: Some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis, Journal of Infection and Immunity 70, 672–678
Buijtels, C.A.M.P., Marrianne, A.B., Graaf, S.D.C., Parkinson, S., Verbrugh, A.H., Petit, L.C.P. and Sooligen, V.D., 2009. Nontuberculous mycobacteria, Zambia, Journal of Emerging Infectious Diseases 15, 242--248
Charette, R., Martineau, G.P., Pigeon, C., Turcotte, C. and Higgins, R., 1989. An outbreak of granulomatous lymphadenitis due to Mycobacterium avium in swine, Journal of Canadian Veterinary Medicine, 675–678
Coetzer, J.A.W., Tustin R.C., 2004. Infectious Diseases of livestock, (Oxford University Press, New York) 3, 1973-1987
Cvetnic, Z., Spicic, S., Jankovic, V.K., Marjanovic, S., Obrovac, M., Benic, M. and Palvlik, I., 2006. Mycobacterium caprae infection in cattle and pigs on one family farm in Croatia: A case report, Veterinarni Medicina 523–531
Griffith, D.E., Aksamit, T., Brown-Elliott, B.A., Catanzaro, A., Daley, C., Gordin, F., Holland, S.M., Horsburgh, R., Huitt, G., Iademarco, M.F., Iseman, M., Olivier, K., Ruoss, S., von Reyn, C.F., Wallace, R.J., Jr., Winthrop, K. and on behalf of the ATS Mycobacterial Diseases Subcommittee, 2007. An Official ATS/IDSA Statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases, American Journal of Respiratory and Critical Care Medicine 175, 367–416
Huang, C., Tsai, Y., Shu, C., Lei, Y., Wang, J., Yu, C., Lee, L., Yang, P. and the TAMI Group, 2009. Clinical significance of isolation of nontuberculous mycobacteria in pulmonary tuberculosis patients, Journal of Respiratory Medicine, 103, 1484–1491
Johansen, T., Olsen, I., Jensen, M., Dahle, U., Holstad, G. and Djonne, B., 2007. New probes used for IS1245 and IS1311 restriction fragment length polymorphism of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis isolates of human and animal origin in Norway, BMC Microbiology, 7, 14
Komijn, R.E., de Haas, P.E., Schneider, M.M., Eger, T., Nieuwenhuijs, J.H., van den Hoek, R.J., Bakker, D., van Zijd Erveld, F.G. and van Soolingen, D., 1999. Prevalence of Mycobacterium avium in slaughter pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates, Journal of Clinical Microbiology 37, 1254--1259
Magezi, S.A.K., 2009. The rain fall outlook for March to May 2009 rainfall season, Ministry of Water and Environment Department of Metrology Uganda, 1–5
Mohamed, A.M., Abou El-Ella, G.A. and Nasr, E.A., 2009. Phenotypic and molecular typing of tuberculous and nontuberculous Mycobacterium species from slaughtered pigs in Egypt, Journal of Veterinary Diagnostic Investigation 21, 48--52
Oloya, J., Muma, J.B., Opuda-Asibo, J., Djønne, B., Kazwala, R. and Skjerve, E., 2007. Risk factors for herd-level bovine-tuberculosis seropositivity in transhumant cattle in Uganda, Journal of Preventive Veterinary Medicine, 80, 318-329
Pavlik, I., Svastova, P., Bartl, J., Dvorska, L. and Rychlik, I., 2000. Relationship between IS901 in the Mycobacterium avium complex strains isolated from birds, animals, humans, and the environment and virulence for poultry, Journal of Clinical and Diagnostic Laboratory Immunology 7, 212--217
Pfyffer, G., Welscher, H., Kissling, P., Cieslak, C., Casal, M., Gutierrez, J. and Rusch- Gerdes, S., 1997. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli, Journal of Clinical Microbiology 35, 364–368
Phillips, P., Bonner, S., Gataric, N., Bai, T., Wilcox, P., Hogg, R., O’Shaughnessy, M. and Montaner, J., 2005. Nontuberculous mycobacterial immune reconstitution syndrome in HIV–infected patients: Spectrum of disease and long–term follow–up, Journal of Clinical Infectious Diseases, 41, 1483–1497
Tirkkonen, T., Pakarinen, J., Moisander, A.M., Maekinen, J., Soini, H. and Ali-Vehmas, T., 2007. High genetic relatedness among Mycobacterium avium strains isolated from pigs and humans revealed by comparative IS1245 RFLP analysis, Journal of Veterinary Microbiology 125, 175–181
Valheim, M., Djonne, B., Heiene, R. and Caugant, D.A., 2001. Disseminated Mycobacterium celatum (Type 3) infection in a domestic ferret (Mustela putorius furo), Journal of Veterinary Pathology, 38, 460–463
Acknowledgements
We would like to thank the Norwegian government for the financial support through the Norwegian School of Veterinary Science and the Quota scholarship scheme. We would also like to thank Kenneth Okanga for the laboratory work in Uganda, Dr. Lunze George, Dr. Asimwe Alani, Dr. Mwanje Gerald, Mr.Willy Yawe and all the Mubende district and sub-county officials for making it easy for us to collect samples in this district.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muwonge, A., Kankya, C., Godfroid, J. et al. Prevalence and associated risk factors of mycobacterial infections in slaughter pigs from Mubende district in Uganda. Trop Anim Health Prod 42, 905–913 (2010). https://doi.org/10.1007/s11250-009-9506-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-009-9506-5