Abstract
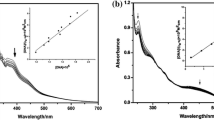
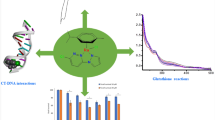
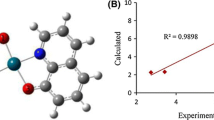
Three oxovanadium complexes, namely [VO(NOSAA)(bpy)] (1) (NOSAA = 2-hydroxy-5-nitrosalicylidene anthranilic acid, bpy = 2,2′-bipyridyl), [VO(NOSAA)(4,4′-dimebpy)] (2) (4,4′-dimebpy = 4,4′-dimethyl-2,2′- bipyridyl), and [VO(NOSAA)(phen)] (3) (phen = 1,10-phenanthroline), have been prepared and characterized. The binding modes and strengths of these complexes with calf thymus DNA (CT-DNA) were studied using various techniques. The chemical nuclease activities and photocleavage reactions of the complexes were also tested. All three complexes interact with CT-DNA through intercalative modes, and complex 3 possesses the largest binding affinity. All three complexes can efficiently cleave pBR322 DNA upon irradiation or under physiological conditions in the presence of H2O2, and complex 3 has the best cleaving ability. In vitro experimental results showed that the three complexes are cytotoxic against myeloma (Ag8.653) and gliomas (U251) cell lines and complex 3 again showed the highest efficacy.







Similar content being viewed by others
References
Thaker BT, Surati KR, Modi CK (2008) Russ J Coord Chem 34:25–33
Bishayee A, Waghray A, Patel MA (2010) Cancer Lett 294:1–12
Gambino Dinorah (2011) Coord Chem Rev 255:2193–2203
Vardatsikos G, Mehdi MZ, Srivastava AK (2009) Int J Mol Med 24(3):303–309
Al-Azemi TF, Vinodh M (2011) Tetrahedron 67:2585–2590
Xin T, Xiaojia H, Jingnan F, Jinli L, Yinzhuang Z (2012) Inorg Chem Commun 15:5–7
Butenko N, Tomaz AI, Nouri O, Escribano E, Moreno V, Gama S, Ribeiro V, Telo JP, Pesssoa JC, Cavaco I (2009) J Inorg Biochem 103:622–632
Sasmal PK, Sounik S, Majumdar R, Dighe RR, Chakravarty AR (2010) Inorg Chem 49:849–859
Julio B, Lorena B, Isabel C, Sandra ML, Helena G, João CP, Julia L, Sebastian T, Patricia E, Virtudes M, Beatriz G, Dinorah G (2011) J Inorg Biochem 105:303–312
Julio B, Guggeri L, Tomaz I, Arrambide G, Navarro M, Costa Pessoa J, Garat B, Gambino D (2009) J Inorg Biochem 103:609–616
Sheela CD, Anitha C, Tharmaraj P, Kodimunthri D (2010) J Coord Chem 63(5):884–893
Sasmal PK, Patra AK, Nethaji M, Chakravarty AR (2007) Inorg Chem 46:11112–11119
Pizarro AM, Sadler PJ (2009) Biochimie 91:1198–1211
Shahabadi N, Kashanian S, Purfoulad M (2009) Spectrochim Acta, Part A 72:757–761
Nataliya B, Ana IT, Ofelia N, Esther E, Virtudes M, Sofia G, Vera R, Joao PT, Joao CP, Isabel C (2009) J Inorg Biochem 103:622–630
Lilia R, Braitbard O, Edit YT (2012) Dalton Trans 41:5241–5247
Niu Y, Liu W, Tian C, Xie M, Gao L, Chen Z, Chen X, Lia L (2007) Eur J Pharmacol 572:213–219
Noblía P, Baran EJ, Otero L, Draper P, Cerecetto H, González M, Piro OE, Castellano EE, Inohara T, Adachi Y, Sakurai H, Gambino D (2004) Eur J Inorg Chem 2:322–329
Mendes IC, Botion LM, Ferreira AVM, Castellano EE, Beraldo H (2009) Inorg Chim Acta 362:414–420
da Maia PIS, Pavan FR, Leite CQF, Lemos S, de Sousa GF, Batista AA, Nascimento OR, Ellena J, Castellano EE, Niquet E, Deflon VM (2009) Polyhedron 28:398–406
McCrate A, Carlone M, Nielsen M, Swavey S (2010) Inorg Chem Commun 13:537–539
Sasmal PK, Patra AK, Chakravarty AR (2008) J Inorg Biochem 102:1463–1472
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen YP, Ma XY, Sui DY (2010) Eur J Pharmacol 645:14–22
D’Urso A, Mammana A, Balaz M, Holmes AE, Berova N, Lauceri R, Purrello R (2009) J Am Chem Soc 131:2046–2047
Guo HW, Lu JZ, Ruan ZG, Zhang YL, Liu YJ, Zang LQ (2012) J Coord Chem 65(2):191–204
Du YF, Lu JZ, Guo HW, Jiang J (2010) Trans Met Chem 35:859–864
Lu JZ, Guo HW, Zeng XD, Zhang YL, Zhao P, Jiang J, Zang LQ (2012) J Inorg Biochem 112:39–48
Lu JZ, Du YF, Guo HW (2011) J Coord Chem 64(7):1229–1239
Li ZP, Xing YH, Zhang YH, Zhou GH (2009) J Coord Chem 62(4):564–576
Efthimiadou EK, Katsaros N, Karaliota A, Psomas G (2007) Bioorg Med Chem Lett 17:1238–1243
Nejo AA, Kolawole GA, Opoku AR, Wolowska J, O’Brien P (2009) Inorg Chem Acta 362:3993–3998
Vaz Serra V, Zamarrón A, Faustino MAF, Iglesias-de la Cruz MC, Blázquez A, Rodrigues JMM (2010) Bioorg Med Chem 18:6170–6178
Tindall MJ, Dyson L, Smallbone K (2012) J Theor Biol 298:107–115
Klein A, Holko P, Ligeza J, Kordowiak AM (2008) Folia Biol 56:115–121
Hazari PP, Pandey AK, Chaturvedi S (2012) Chem Biol Drug Des 79(2):223–234
Acknowledgments
We gratefully acknowledge financial support for this work by the Science and technology Research Project of Guangdong Province (No.2012B031800431), P. R. China and the University Student Innovating Program Foundation of Guangdong Province,P. R. China (No. 1057310014).
Conflict of interest
The authors have declared that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, J., Guo, J., Sang, W. et al. Mixed-ligand oxovanadium complexes incorporating Schiff base ligands: synthesis, DNA interactions, and cytotoxicities. Transition Met Chem 38, 481–488 (2013). https://doi.org/10.1007/s11243-013-9714-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9714-8