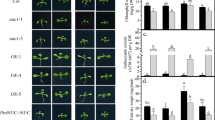
Abstract
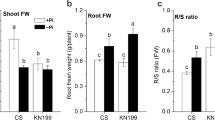
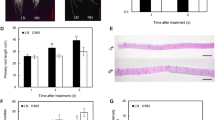
One of the most important micronutrients required for normal development of plants is phosphorus. In terms of phosphorus starvation, plants have developed plasticity for overcoming stress, expressed in the formation of shortened and thickened cluster roots. Both phytohormones auxin and strigolactone participate in the plant response associated with the phosphate signaling. The present study revealed the interaction and correlation between auxin and strigolactones in Medicago truncatula plants with modified auxin transport under extreme conditions of phosphate deficiency and excess. In general, drastic changes in the root architecture were observed in both overexpression and RNAi-lines. In terms of phosphate deprivation the number of lateral roots has gained and the length of main root was reduced. Relative transcript level of MtLAX3 gene and the two strigolactone genes MtMAX2 and MtMAX3 was upregulated in OE/RNAi-lines and wild type plants in terms of phosphorus starvation compare to same lines in normal condition.





Similar content being viewed by others
References
Alcântara A, Morgado RS, Silvestre S, Marques da Silva J, Bernardes da Silva A, Fevereiro P, Araújo SS (2015) A method to identify early-stage transgenic Medicago truncatula with improved physiological response to water deficit. Plant Cell Tiss Organ Cult 122:605–616
Araújo SS, Balestrazzi A, Faè M, Morano M, Carbonera D, Macovei A (2016) MtTdp2α-overexpression boosts the growth phase of Medicago truncatula cell suspension and increases the expression of key genes involved in antioxidant response and genome stability. Plant Cell Tiss Organ Cult 127:675–680
Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029
Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006) Characterisation of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45:982–993
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Opin Plant Biol 16:553–563
Beveridge CA (2000) Long-distance signalling and mutational analysis of branching in pea. Plant Growth Regul 32:193–203
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
d’Erfurth I, Cosson V, Eschstruth A, Lucas H, Kondorosi A, Ratet P (2003) Efficient transposition of the Tnt1 tobacco retrotransposon in the model legume Medicago truncatula. Plant J 34:95–106
De Cuyper C, Fromentin J, Yocgo RE, De Keyser A, Guillotin B, Kunert K, Boyer FD, Goormachtig S (2014) From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J Exp Bot 66(1):137–146
Duque AS, López-Gómez M, Kráčmarová J, Gomes CN, Araújo SS, Lluch C, Fevereiro P (2016) Genetic engineering of polyamine metabolism changes Medicago truncatula responses to water deficit. Plant Cell Tiss Organ Cult 127:681–690
Giehl RFH, Gruber BD, Wiren N (2014) It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Botany 65:769–778
Iantcheva A, Chabaud M, Cosson V, Barascud M, Schutz B, Primard-Brisset C, Durand P, Barker DG, Vlahova M, Ratet P (2009) Osmotic shock improves Tnt1 transposition frequency Medicago truncatula cv. Jemalong during vitro regeneration. Plant Cell Rep 28:1563–1572
Iantcheva A, Revalska M, Zehirov G, Vassileva V (2013) Agrobacterium-mediated transformation of Medicago truncatula cell suspension culture provides a system for functional analysis. In Vitro Cell Dev Biol Plant 50:149–157
Jacob C, Carrasco B, Schwember AR (2016) Advances in breeding and biotechnology of legume crops. Plant Cell Tiss Organ Cult 127:561–584
Jiang L, Maathys C, Marquez-Garcia B, De Cuyper C, Smet L, De Keyser A, Boyer FD, Beeckman T, Depuydt S, Goormachtig S (2015) Strigolactones spatially influence lateral root development through the cytokinin signaling network. J Exp Bot 67(1):379–389
Kapulnik Y, Delaux PM, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier JP, Bécard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, Koltai H (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233:209–216
Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C (2011) Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 155:974–987
Lavenus J, Goh T, Roberts I, Guyomarch S, Lukas M, Smet ID, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18(8):450–458
Maathuis FJ (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258
Maathys C, Walton A, Struk S, Stes E, Boyer FD, Gevaert K, Goormachtig S (2016) The whats, the wheres and the hows of strigolactone action in the roots. Planta 243:1327–1337
Mayzlish-Gati E, De-Cuyper C, Goormachtig S, Beeckman T, Vuylsteke M, Brewer PB, Beveridge CA, Yermiyahu U, Kaplan Y, Enzer Y, Wininger S, Resnick N, Cohen M, Kapulnik Y, Koltai H (2012) Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol 160:1329–1341
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138:2061–2074
Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS (2013) Responses of root architecture development to low phosphorus availability: a review. Ann Bot 112:391–408
Nolan KE, Rose RJ, Gorst JR (1989) Regeneration of Medicago truncatula from tissue culture: increased somatic embryogenesis using explants from regenerated plants. Plant Cell Rep 8:278–281
Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U (2006) Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol 140:1496–1506
Orłowska A, Igielski R, Łagowska K, Kępczyńska E (2017) Identification of LEC1, L1L and polycomb repressive complex 2 genes and their expression during the induction phase of Medicago truncatula Gaertn. Somatic embryogenesis. Plant Cell Tiss Organ Cult 129:119–132
Parry G, Delbarre A, Marchant A, Swarup R, Perrot-Rechenmann C, Bennett M (2001) Physiological characterization of a novel class of auxin influx carrier inhibitors. Plant J 25:399–406
Péret B, De Rybel B, Casimiro I, Benkova E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14:399–408
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450
Péret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L (2014) Root architecture responses: in search for phosphate. Am Soc Plant Biol. https://doi.org/10.1104/pp.114.244541
Perez-Torres CA, Lopes-Bucio J, Cruz-Ramirez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20:3258–3272
Rasmussen A, Depuydt S, Goormachtig S, Geelen D (2013) Strigolactones fine tune the root system. Planta 238:615–626
Remblière C, Fournier J, de Carvalho-Niebel F, Chabaud M (2018) A simple Agrobacterium tumefaciens-mediated transformation method for rapid transgene expression in Medicago truncatula root hairs. Plant Cell Tiss Organ Cult 132:181–190
Revalska M, Vassileva V, Goormachtig S, Van Hautegem T, Ratet P, Ianctheva A (2011) Recent progress in development of a Tnt1 functional genomics platform for the model legumes Medicago truncatula and Lotus japonicus in Bulgaria. Curr Gen 12:147–152
Revalska M, Zehirov G, Vassileva V, Iantcheva AV (2015) Is the auxin influx carrier LAX3 essential for plant growth and development in the model plants Medicago truncatula, Lotus japonicus and Arabidopsis thaliana? Biotechnol Biotechnol Eq 29(4):786–797
Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R, Verstappen F, Bouwmeester H (2011) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 155:721–734
Saini S, Sharma I, Kaur N, Pati PK (2013) Auxin: a master regulator of plant root development. Plant Cell Rep 32:741–757
Sanchez CA (2015) Phosphorus. In: Barker AV, Pilbeam DJ (eds) Handbook of plant nutrition, Taylor & Francis Group, Boca Raton, pp 51–91
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11:e1001474
Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC (2007) Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol 143:697–706
Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15:2648–2653
Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R et al (2004) Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16:3069–3083
Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y et al (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10:946–954
Tadege M, Ratet P, Mysore KS (2005) Insertional mutagenesis: a Swiss Army knife for functional genomics of M. truncatula. Trends Plant Sci 10:229–235
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95:707–735
Yang YD, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) A 1 influx carrier prote. Curr Biol 16:1123–1127
Young ND, Udvardi M (2009) Translating Medicago truncatula genomics to crop legumes. Curr Opin Plant Biol 12:193–201
Acknowledgements
This study was supported by a Grant from National Science Fund at the Ministry of Education and Science of the Republic of Bulgaria, Project DM06/2 “Hormonal crosstalk between auxin and strigolactones in Medicago truncatula plants with modified auxin transport”. The authors are grateful for technical help of Kety Krastanova.
Author information
Authors and Affiliations
Contributions
MR designed the research and performed the experiments. MR and AI analysed the data and results. MR drafted the manuscript. AI revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Sergio J. Ochatt.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Figure 1
. Relative expression level of MtLAX3 in T0 and T1 M. truncatula MtLAX3-OE and MtLAX3-RNAi compared to WT plants (JPG 2522 KB)

Supplemental Figure 2
. Descriptive statistics of Two-Way ANOVA and “Interaction plot” between the two factors “treatment” and “genotype” for main root length (MRL) (JPG 7443 KB)

Supplemental Figure 3
. Descriptive statistics of Two-Way ANOVA and “Interaction plot” between the two factors “treatment” and “genotype” for lateral root number (LRN) (JPG 7243 KB)

Supplemental Figure 4
. Descriptive statistics of Two-Way ANOVA and “Interaction plot” between the two factors “treatment” and “genotype” for relative expression level of MtLAX3 (JPG 5269 KB)

Supplemental Figure 5
. Descriptive statistics of Two-Way ANOVA and “Interaction plot” between the two factors “treatment” and “genotype” for relative expression level of MtMAX2 (JPG 5315 KB)

Supplemental Figure 6
. Descriptive statistics of Two-Way ANOVA and “Interaction plot” between the two factors “treatment” and “genotype” for relative expression level of MtMAX3 (JPG 5587 KB)
Rights and permissions
About this article
Cite this article
Revalska, M., Iantcheva, A. Pi-starvation is mitigated in Medicago truncatula plants with upregulated auxin transport through auxin–strigolactone interaction. Plant Cell Tiss Organ Cult 133, 405–415 (2018). https://doi.org/10.1007/s11240-018-1393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1393-x