Abstract
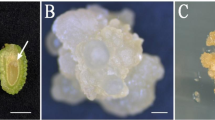
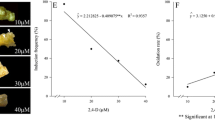
In this study, mature zygotic embryos, plant growth regulators, and various media were tested with the aim of developing an efficient regeneration system for plantlets of the bamboo species Dendrocalamus hamiltonii. Callus formation was induced in explants cultured in Murashige and Skoog (MS) medium supplemented with 1.0–3.0 mg/l 2,4-dichlorophenoxyacetic acid. Optimal shoot differentiation and subsequent shoot growth were also obtained in MS medium supplemented with 2 mg/l benzyladenine, 1 mg/l kinetin, and 1 mg/l naphthaleneacetic acid. Root induction was enhanced by the addition of 5 mg/l indole-3-butyric acid to the culture medium. Histological analysis revealed that both somatic embryogenesis and organogenesis were induced during callus initiation, shoot differentiation, and the development of plantlets from the mature zygotic embryos. Our data provide a useful basis for developing culture protocols for the regeneration of bamboo plants.




Similar content being viewed by others
Notes
Embryogenic callus was initiated and plant regeneration achieved by culturing spikelets and seeds explants of Bambusa multiplex. Wu’s NB medium supplemented with 500 mg/l proline, 500 mg/l glutamine, 300 mg/l casein hydrolysate, 30 g/l sucrose, 8 g/l carrageenan, and 4 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D) was found to be the most appropriate medium for callus induction. However, only a few calli induced from spikelets germinated, whereas calli induced from seeds had a regeneration rate of up to 80%. The optimal pre-differentiation medium for seed embryoids was MS (Murashige and Skoog 1962) basal medium supplemented with 3 mg/l benzylaminopurine and 3 mg/l kinetin. Plantlets were rooted in MS supplemented with 2 mg/l naphthaleneacetic acid.
The genotypical differences in the ability of anthers to be cultured and the inheritance of this ability were investigated in seven keng rice (Oryza sativa subsp. keng) varieties and their hybrids. The induction basal medium for anther culture was NB medium (N6 macrosalts, B5 microsalts, and organic compounds), while MS basal medium was used for shoot differentiation. The results showed that the genotypic variances in general and specific combining abilities of callus induction frequencies and shoot differentiation frequencies were significant at the 1% level. The results of this study indicated that it was possible to transfer genes of anthers showing a high culture ability from one genotype to another by crossing.
The effect of HB medium supplemented with 2 mg/l 2,4-D on callus induction and maintenance in 25 wheat genotypes in comparison with that of N6 medium. The results showed that HB medium was more effective in terms of both callus induction and maintenance.
References
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Godbole S, Sood A, Thakur R, Sharma M, Ahuja PS (2002) Somatic embryogenesis and its conversion into plantlets in a multipurpose bamboo, Dendrocalamus hamiltonii Nees et Arn.Ex Munro. Curr Sci 83:885–889
Hassan AE, Debergh P (1987) Embryogenesis and plantlet development in the bamboo Phyllostachys viridis (Young) McClure. Plant Cell Tissue Organ Cult 10:73–77
Huang LC, Murashige T (1983) Tissue culture investigation of bamboo I: callus culture of Bambusa, Phyllostachys and Sasa. Bot Bull Acad Sin 24:31–52
Huang LC, Huang BL, Chen WL (1989) Tissue culture investigations of bamboo IV: organogenesis leading to adventitious shoots and plants in excised shoot apices. Environ Exp Bot 29:307–315
Johansen DA (1940) Plant microtechnique, 1st edn. McGraw-Hill, New York
Keng PC (1996) Flora of China, vol 9, number 1, 1st edn. Science Press, Beijing
Larkin PJ, Scowcroft WP (1981) Somaclonal variation-novel source of variability from cell culture for plant improvement. Theor Appl Genet 60:197–214
Liu JH, Shi JC (1996) Studies on selection of valuable somaclonal mutants in silage maize. Acta Bot Sin 38:839–842
Liu L, Fan XL, Zhang JW, Yan ML, Bao MZ (2009) Long-term cultured callus and the effect factor of high-frequency plantlet regeneration and somatic embryogenesis maintenance in Zoysia japonica. In Vitro Cell Dev Biol Plant 45:673–680
Mehta U, Rao IVR, Ram HYM (1982) Somatic embryogenesis in bamboo. In: Proc 5th Intl Cong Plant Tissue and Cell Culture. pp 109–110
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Rajneesh KA, Janhvi M, Shyamal KN (2009) Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. Ex Munro: production of genetically uniform plants and field evaluation. Acta Physiol Plant 31:961–967
Rao IU, Rao IVR, Narang V (1985) Somatic embryogenesis and regeneration of plants in the bamboo Dendrocalamus strictus. Plant Cell Rep 4:191–194
Rout GR, Das P (1994) Somatic embryogenesis and in vitro flowering of 3 species of bamboo. Plant Cell Rep 13:683–686
Sun GZ, Ma MQ, Zhang YQ, Xie XL, Cai JF, Li XP, Yin ZM, Qin J, Zhang R, Xu D, Yang JY, Wang HB (1999) A suitable medium for callus induction and maintenance for wheat. J Hebei Agric Sci 3:224–226
Tsay HS, Yeh CC, Hsu JY (1990) Embryogenesis and plant regeneration from anther culture of bamboo (Sinocalamus latiflora (Munro) McClure). Plant Cell Rep 9:349–351
Woods SH, Phillips GC, Woods JE, Collins GB (1992) Somatic embryogenesis and plant regeneration from zygotic embryo explants in Mexican weeping bamboo, Otatea acuminata aztecorum. Plant Cell Rep 11:257–261
Wu CY, Chen Y (1987) A study on the genotypical differences in anther culture of Keng rice (Oryza sativa subsp. keng). Acta Genet Sin 14:168–174
Yeh M, Chang WC (1986a) Plant regeneration through somatic embryogenesis in callus culture of green bamboo (Bambusa oldhamii Munro). Theor Appl Genet 73:61–163
Yeh M, Chang WC (1986b) Somatic embryogenesis and subsequent plant regeneration from inflorescence callus of Bambusa beecheyana Munro var. beecheyana. Plant Cell Rep 5:409–411
Yeh M, Chang WC (1987) Plant regeneration via somatic embryogenesis in mature embryo-derived callus culture of Sinocalamus latiflora (Munro) McClure. Plant Sci 51:93–96
Yuan JL, Gu XP, Li LB, Yue JJ, Yao N, Guo GP (2009) Induction and plantlet regeneration of Bambusa multiplex. Sci Silvae Sin 3:35–40
Acknowledgments
We thank Dr. Xuerong Shi for his critical reading of the manuscript, and Dr. Lichun Huang at Taiwan Academia Sinica for providing technical assistance during the bamboo tissue culture. This work was supported by research grants from the Science and Technology Department of Zhejiang Province, China [grant no. 2006C12008].
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, N., Fang, W., Shi, Y. et al. Somatic embryogenesis and organogenesis in Dendrocalamus hamiltonii . Plant Cell Tiss Organ Cult 103, 325–332 (2010). https://doi.org/10.1007/s11240-010-9783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9783-8