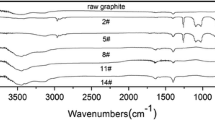
This is the first demonstration of the feasibility of using the interaction of paramagnetic sites of mechanochemically nanostructured graphite particles with aliphatic alcohols (ethanol and octanol) to change the properties of graphenes formed in the liquid-phase exfoliation of thus modified samples of nanostructured graphite. The presence of various functional groups was confirmed spectroscopically. Raman spectroscopy was used to show that treatment of mechanochemically nanostructured graphite with alcohols may lead to graphenes with fewer defects. The graphene nanoparticles from the resultant dispersions have a tendency to agglomerate upon the removal of the solvent, which increases due to the presence of functional groups on their surface.






Similar content being viewed by others
References
P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 110, No. 1, 268-320 (2010).
A. C. Grimsdale, K. L. Chan, R. E. Martin, et al., Chem. Rev., 109, No. 3, 897-1091 (2009).
X.-F. Zhang and Q. Xi, Carbon, 49, No. 12, 3842-3850 (2011).
Z. Liu, Q. Liu, Y. Huang, et al., Adv. Mater., 20, No. 20, 3924-3930 (2008).
E. Kymakis, I. Alexandrou, and G. A. J. Amaratunga, J. Appl. Phys., 93, No. 3, 1764-1768 (2003).
V. Georgakilas, M. Otyepka, A. B. Bourlinos, et al., Chem. Rev., 112, 6156-6214 (2012).
K. S. Subrahmanyam, A. Ghosh, A. Gomathi, et al., Nanosci. Nanotechnol. Lett., 1, No. 1, 28-31 (2009).
Y. Liu, J. Zhou, X. Zhang, et al., Carbon, 47, No. 13, 3113-3121 (2009).
A. Hirsch, J. M. Englert, and F. Hauke, Accounts Chem. Res., 46, No. 1, 87-96 (2013).
Ch. K. Chua and M. Pumera, Chem. Soc. Rev., 42, No. 8, 3222-3233 (2013).
V. Georgakilas, J. N. Tiwari, K. C. Kemp, et al., Chem. Rev., 116, No. 9, 5464-5519 (2016).
S. P. Lonkar, Y. S. Deshmukh, and A. A. Abdala, Nano Res., 8, No. 4, 1039-1074 (2015).
O. Yu. Posudievsky, O. A. Khazieieva, V. V. Cherepanov, et al., J. Nanopart. Res., 15, No. 11, 2046 (2013).
J. Kausteklis, P. Cevc, C. Arčon, et al., Phys. Rev. B, 84, No. 12, 125406 (2011).
O. Yu. Posudievsky, O. A. Khazieieva, O. A. Kozarenko, et al., Teor. Éksp. Khim., 52, No. 1, 3-8 (2016). [Theor. Exp. Chem., 52, No. 1, 2-9 (2016) (English translation).]
X. Chen, H. Chen, J. Guan, et al., Nanoscale, 9, 5615-5623 (2017).
S. Chen, R. Xu, J. Liu, et al., Adv. Mater., 1804810 (2019).
U. Khan, A. O’Neill, M. Lotya, et al., Small, 6, No. 7, 864-871 (2010).
U. Khan, H. Porwal, A. O’Neill, et al., Langmuir, 27, 9077-9082 (2011).
Y. Si and E. T. Samulski, Nano Lett., 8, No. 6, 1679-1682 (2008).
M. Fan, C. Zhu, J. Yang, et al., Electrochim. Acta, 216, 102-109 (2016).
L. Niu, J. N. Coleman, H. Zhang, et al., Small, 12, No. 3, 272-293 (2016).
D. Bischoff, J. Güttinger, S. Dröscher, et al., J. Appl. Phys., 109, No. 7, 073710 (2011).
L. Cançado, M. Pimenta, B. Neves, et al., Phys. Rev. Lett., 93, No. 24, 247401 (2004).
D. Yoon, H. Moon, H. Cheong, et al., J. Korean Phys. Soc., 55, No. 3, 1299-1303 (2009).
This work was carried out with the support of the Targeted Scientific Research Initiative of the Ukrainian Science and Technology Center (USTC) and National Academy of Sciences of Ukraine as well as the Targeted Joint Basic Research Program of the National Academy of Sciences of Ukraine on Basic Problems in the Creation of New Nanomaterials and Nanotechnologies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 55, No. 2, pp. 88-94, March-April, 2019.
Rights and permissions
About this article
Cite this article
Posudievsky, O.Y., Kondratyuk, A.S., Cherepanov, V.V. et al. Modified Graphenes Prepared by the Interaction of Mechanochemically Nanostructured Graphite with Water and Aliphatic Alcohols. Theor Exp Chem 55, 96–102 (2019). https://doi.org/10.1007/s11237-019-09599-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-019-09599-1