Abstract
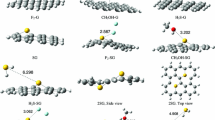
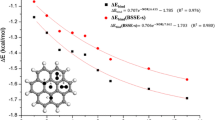
Density functional theory (DFT) calculations were used to examine the binding strength of one and two methane molecule(s) with graphene (62 and 186 carbon atoms) and model systems of aromatic hydrocarbons (benzene, pyrene, and coronene). We explored different possibilities of binding modes of methane such as one, two, and three C-H interacting with small π-systems. Two methane molecules were considered to bind from the same as well as opposite sides of the plane of benzene and other π-systems including graphene models. Our results show that methane molecule prefers to bind with three C-H…π interactions with all the π-systems except benzene. The preference of tripod configuration of methane on the surface of graphene systems strongly agrees with the neutron diffraction experiment of methane on graphitized carbon black. The binding strength is almost doubled by increasing the number of methane molecules from one to two. Importantly, two methane molecules prefer to bind on the same side rather than opposite sides of the plane of graphene due to stabilizing CH…HC interactions between them in addition to six C-H…π interactions. Interestingly, binding strength contributions from CH…HC interactions (approx. 0.4–0.5 kcal/mol) of two methane molecules on the surface are analogous to methane dimer complex free from the surface of graphene. C-H stretching frequency shifts, bond lengths, and binding distances support the presence of CH…HC interactions between two methane molecules. Structures of complexes, binding energies, and C-H stretching frequency shifts agree with available experimental data.









Similar content being viewed by others
References
Bowers GM, Loring JS, Schaef HT, Walter ED, Burton SD, Hoyt DW, Cunniff SS, Loganathan N, Kirkpatrick RJ (2018) Interaction of hydrocarbons with clays under reservoir conditions: in situ infrared and nuclear magnetic resonance spectroscopy and X-ray diffraction for expandable clays with variably wet supercritical methane. ACS Earth Space Chem 2:640–652
Li W, Liu Y, Xiao S, Zhang Y, Chai L (2018) An investigation of the underlying evolution of shale gas research’s domain based on the co-word network. Sustainability 10:164
Wang Q, Chen X, Jha AN, Rogers H (2014) Natural gas from shale formation—the evolution, evidences and challenges of shale gas revolution in United States. Renew Sust Energ Rev 30:1–28
Wang F, Liu G-Q, Meng H-L, Guo G, Luo S-J, Guo R-B (2016) Improved methane hydrate formation and dissociation with nanosphere-based fixed surfactants as promoters. ACS Sustain Chem Eng 4:2107–2113
Collett T, Bahk J-J, Baker R, Boswell R, Divins D, Frye M, Goldberg D, Husebø J, Koh C, Malone M, Morell M, Myers G, Shipp C, Torres M (2015) Methane hydrates in nature—current knowledge and challenges. J Chem Eng Data 60:319–332
Jiao Y, Wang L, Zhang L, An W, Wang W, Zhou W, Tadé MO, Shao Z, Bai J, Li S-D (2018) Direct operation of solid oxide fuel cells on low-concentration oxygen-bearing coal-bed methane with high stability. Energy Fuel 32:4547–4558
Karacan COR, Ruiz FA, Cotè M, Phipps S (2011) Coal mine methane; a review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction. Int J Coal Geol 86:121–156
Zhou Y, Fu Q, Shen Y, Sun W, Zhang D, Li D, Yan H (2016) Upgrade of low-concentration oxygen-bearing coal bed methane by a vacuum pressure swing adsorption process: performance study and safety analysis. Energy Fuel 30:1496–1509
Vielstädte L, Haeckel M, Karstens J, Linke P, Schmidt M, Steinle L, Wallmann K (2017) Shallow gas migration along hydrocarbon wells—an unconsidered, anthropogenic source of biogenic methane in the North Sea. Environ Sci Technol 51:10262–10268
Chen JJ, Li WW, Li XL, Yu HQ (2012) Improving biogas separation and methane storage with multilayer graphene nanostructure via layer spacing optimization and lithium doping: a molecular simulation investigation. Environ Sci Technol 46:10341–10348
Dhingra R, Christensen ER, Liu Y, Zhong BO, Wu C-F, Yost MG, Remais JV (2011) Greenhouse gas emission reductions from domestic anaerobic digesters linked with sustainable sanitation in rural China. Environ Sci Technol 45:2345–2352
Lee H-H, Ahn S-H, Nam B-U, Kim B-S, Lee G-W, Moon D, Shin HJ, Han KW, Yoon J-H (2012) Thermodynamic stability, spectroscopic identification, and gas storage capacity of CO2–CH4–N2 mixture gas hydrates: implications for landfill gas hydrates. Environ Sci Technol 46:4184–4190
Zhang X, Sun Y, Su W, Wang X (2016) Adsorption equilibria of C1–C4 from natural gas on graphene sheets. J Chem Eng Data 61:1667–1675
Zhu ZW, Zheng QR (2016) Methane adsorption on the graphene sheets, activated carbon and carbon black. Appl Therm Eng 108(Supplement C):605–613
Chouhan RK, Ulman K, Narasimhan S (2015) Graphene oxide as an optimal candidate material for methane storage. J Chem Phys 143:044704
Mahmoudian L, Rashidi A, Dehghani H, Rahighi R (2016) Single-step scalable synthesis of three-dimensional highly porous graphene with favorable methane adsorption. Chem Eng J 304(Supplement C):784–792
Patel P (2017) Chemical sensing: looking for methane leaks. C&EN Global Enterprise 95:19–22
Patel P (2017) Monitoring methane. ACS Cent Sci 3:679–682
Yang N, Yang D, Zhang G, Chen L, Liu D, Cai M, Fan X (2018) The effects of graphene stacking on the performance of methane sensor: a first-principles study on the adsorption, band gap and doping of graphene. Sensors (Basel, Switzerland) 18:422
Srinivas G, Guo ZX (2015) Graphene-based materials: synthesis and gas sorption, storage and separation. Prog Mater Sci 69:1–60
García-Hernández E, Salazar-García E, Shakerzadeh E, Chigo-Anotac E (2020) Effect of dehydrogenated hydrocarbon doping on the electronic properties of graphene-type nanosheets. Phys Lett A 384:126702
Chigo-Anota E, Alejandro MA, Hernández AB, Torres JJS, Castro M (2016) Long range corrected-wPBE based analysis of the H2O adsorption on magnetic BC3 nanosheets. RSC Adv 6:20409–20421
Hernández JMG, Anota EC, de la Cruz MRT, Melchor MG, Gregorio Hernández Cocoletzi GH (2012) First principles studies of the graphene-phenol interactions. J Mol Model 18:3857–3866
Dinadayalane T, Leszczynska D, Leszczynski J (2012) Graphene: properties, biomedical applications and toxicity. In: Puzyn T, Leszczynski J (eds) Towards efficient designing of safe nanomaterials: innovative merge of computational approaches and experimental techniques, vol 25. Royal Society of Chemistry, Cambridge, pp 1–26
Dinadayalane TC, Leszczynski J (2016) Fundamental structural, electronic, and chemical properties of carbon nanostructures: graphene, fullerenes, carbon nanotubes, and their derivatives. In: Leszczynski J (ed) Handbook of computational chemistry. Springer, Dordrecht, pp 1–84
Feng S, dos Santos MC, Carvalho BR, Lv R, Li Q, Fujisawa K, Elías AL, Lei Y, Perea-López N, Endo M, Pan M, Pimenta MA, Terrones M (2016) Ultrasensitive molecular sensor using N-doped graphene through enhanced Raman scattering. Sci Adv 2:e1600322
Wu Z, Chen X, Zhu S, Zhou Z, Yao Y, Quan W, Liu B (2013) Room temperature methane sensor based on graphene nanosheets/polyaniline nanocomposite thin film. IEEE Sensors J 13:777–782
Pykal M, Jurečka P, Karlický F, Otyepka M (2016) Modelling of graphene functionalization. Phys Chem Chem Phys 18:6351–6372
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2000) The magnitude of the CH/π interaction between benzene and some model hydrocarbons. J Am Chem Soc 122:3746–3753
Ramezanpour M, Leung SSW, Delgado-Magnero KH, Bashe BYM, Thewalt J, Tieleman DP (2016) Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim Biophys Acta Biomembr 1858:1688–1709
Ringer AL, Figgs MS, Sinnokrot MO, Sherrill CD (2006) Aliphatic C−H/π interactions: methane−benzene, methane−phenol, and methane−indole complexes. J Phys Chem A 110:10822–10828
Shibasaki K, Fujii A, Mikami N, Tsuzuki S (2006) Magnitude of the CH/π interaction in the gas phase: experimental and theoretical determination of the accurate interaction energy in benzene-methane. J Phys Chem A 110:4397–4404
Tsuzuki S, Honda K, Fujii A, Uchimaru T, Mikami M (2008) CH/π interactions in methane clusters with polycyclic aromatic hydrocarbons. Phys Chem Chem Phys 10:2860–2865
Karthikeyan S, Ramanathan V, Mishra BK (2013) Influence of the substituents on the CH···π interaction: benzene-methane complex. J Phys Chem A 117:6687–6694
Smith DGA, Patkowski K (2013) Interactions between methane and polycyclic aromatic hydrocarbons: a high accuracy benchmark study. J Chem Theory Comput 9:370–389
Paytakov G, Dinadayalane T, Leszczynski J (2015) Toward selection of efficient density functionals for van der Waals molecular complexes: comparative study of C–H···π and N–H···π interactions. J Phys Chem A 119:1190–1200
Vekeman J, G. Cuesta I, Faginas-Lago N, Wilson J, Sánchez-Marín J, Sánchez de Merás A (2018) Potential models for the simulation of methane adsorption on graphene: development and CCSD(T) benchmarks. Phys Chem Chem Phys 20:25518–25530
Qiu N-X, Xue Y, Guo Y, Sun W-J, Chu W (2012) Adsorption of methane on carbon models of coal surface studied by the density functional theory including dispersion correction (DFT-D3). Comput Theor Chem 992:37–47
Morita S-i, Fujii A, Mikami N, Tsuzuki S (2006) Origin of the attraction in aliphatic C−H/π interactions: infrared spectroscopic and theoretical characterization of gas-phase clusters of aromatics with methane. J Phys Chem A 110:10583–10590
Daggag D, Lazare J, Dinadayalane T (2019) Conformation dependence of tyrosine binding on the surface of graphene: bent prefers over parallel orientation. Appl Surf Sci 483:178–186
Daggag D, Dorlus T, Dinadayalane T (2019) Binding of histidine and proline with graphene: DFT study. Chem Phys Lett 730:147–152
Daggag D, Lazare J, Dinadayalane T (2019) Data related to conformation dependence of tyrosine binding on the surface of graphene: bent prefers over parallel orientation. Data Brief 26:104420
Pandit S, De M (2017) Interaction of amino acids and graphene oxide: trends in thermodynamic properties. J Phys Chem C 121:600–608
Vovusha H, Sanyal S, Sanyal B (2013) Interaction of nucleobases and aromatic amino acids with graphene oxide and graphene flakes. J Phys Chem Lett 4:3710–3718
Nassef HM, Hagar M, Malek Z, Othman AM (2018) Uptake of tyrosine amino acid on nano-graphene oxide. Materials 11:68
Plevin MJ, Bryce DL, Boisbouvier J (2010) Direct detection of CH/π interactions in proteins. Nat Chem 2:466
Jenness GR, Karalti O, Jordan KD (2010) Benchmark calculations of water–acene interaction energies: extrapolation to the water–graphene limit and assessment of dispersion–corrected DFT methods. Phys Chem Chem Phys 12:6375–6381
Rubeš M, Bludský O (2009) DFT/CCSD(T) investigation of the interaction of molecular hydrogen with carbon nanostructures. ChemPhysChem 10:1868–1873
Rubeš M, Nachtigall P, Vondrášek J, Bludský O (2009) Structure and stability of the water−graphite complexes. J Phys Chem C 113:8412–8419
Lazar P, Karlický F, Jurečka P, Kocman M, Otyepková E, Šafářová K, Otyepka M (2013) Adsorption of small organic molecules on graphene. J Am Chem Soc 135:6372–6377
Podeszwa R (2010) Interactions of graphene sheets deduced from properties of polycyclic aromatic hydrocarbons. J Chem Phys 132:044704
Medeiros PVC, Gueorguiev GK, Stafström S (2015) Bonding, charge rearrangement and interface dipoles of benzene, graphene, and PAH molecules on Au(111) and Cu(111). Carbon 81:620–628
Vidali G, Ihm G, Kim H-Y, Cole MW (1991) Potentials of physical adsorption. Surf Sci Rep 12:135–181
Do DD, Do HD (2005) Evaluation of 1-site and 5-site models of methane on its adsorption on graphite and in graphitic slit pores. J Phys Chem B 109:19288–19295
Albesa AG, Llanos JL, Vicente JL (2008) Comparative study of methane adsorption on graphite. Langmuir 24:3836–3840
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, Van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181:1477–1489
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Gao T, Li H, Li W, Li L, Fang C, Li H, Hu L, Lu Y, Su Z-M (2016) A machine learning correction for DFT non-covalent interactions based on the S22, S66 and X40 benchmark databases. J Cheminform 8:24
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2004) Erratum: “Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes” [J Chem Phys (2003) 119:12129]. J Chem Phys 121:11507–11507
Barone V, Hod O, Peralta JE, Scuseria GE (2011) Accurate prediction of the electronic properties of low-dimensional graphene derivatives using a screened hybrid density functional. Acc Chem Res 44:269–279
Dinadayalane TC, Leszczynski J (2013) Comparative theoretical study on the positional preference for functionalization of two OH and SH groups with (5,5) armchair SWCNT. J Phys Chem C 117:14441–14450
Herath D, Dinadayalane T (2017) Computational investigation of double nitrogen doping on graphene. J Mol Model 24:26
Wróbel J, Kurzydłowski KJ, Hummer K, Kresse G, Piechota J (2009) Calculations of ZnO properties using the Heyd-Scuseria-Ernzerhof screened hybrid density functional. Phys Rev B 80:155124
Wu J, Hagelberg F, Dinadayalane TC, Leszczynska D, Leszczynski J (2011) Do Stone–Wales defects alter the magnetic and transport properties of single-walled carbon nanotubes? J Phys Chem C 115:22232–22241
NIST Computational Chemistry Comparison and Benchmark Database (2019) National Institute of Standards and Technology (NIST). http://cccbdb.nist.gov.
Rayment T, Thomas RK, Bomchil G, White JW (1981) The structure and properties of methane adsorbed on graphitized carbon black determined by neutron diffraction. Mol Phys 43:601–620
Danovich D, Shaik S, Neese F, Echeverria J, Aullon G, Alvarez S (2013) Understanding the nature of the CH···HC interactions in alkanes. J Chem Theory Comput 9:1977–1991
Wang C, Mo Y, Wagner JP, Schreiner PR, Jemmis ED, Danovich D, Shaik S (2015) The self-association of graphane is driven by London dispersion and enhanced orbital interactions. J Chem Theory Comput 11:1621–1630
Rösel S, Quanz H, Logemann C, Becker J, Mossou E, Cañadillas-Delgado L, Caldeweyher E, Grimme S, Schreiner PR (2017) London dispersion enables the shortest intermolecular hydrocarbon H···H contact. J Am Chem Soc 139:7428–7431
Schreiner P, Chernish L, Gunchenko P, Tikhonchuk E, Hausmann H, Serafin M, Schlecht S, Dahl J, Carlson R, Fokin A (2011) Overcoming lability of extremely long alkane carbon-carbon bonds through dispersion forces. Nature 477:308–311
Grimme S, Schreiner PR (2011) Steric crowding can stabilize a labile molecule: solving the hexaphenylethane riddle. Angew Chem Int Ed 50:12639–12642
Fokin A, Gerbig D, Schreiner P (2011) σ/σ- and π/π-interactions are equally important: multilayered graphanes. J Am Chem Soc 133:20036–20039
Fokin AA, Chernish LV, Gunchenko PA, Tikhonchuk EY, Hausmann H, Serafin M, Dahl JEP, Carlson RMK, Schreiner PR (2012) Stable alkanes containing very long carbon–carbon bonds. J Am Chem Soc 134(33):13641–13650
Palacios T, Hsu A, Wang H (2010) Applications of graphene devices in RF communications. IEEE Commun Magaz 48:122–128
Nevius MS, Conrad M, Wang F, Celis A, Nair MN, Taleb-Ibrahimi A, Tejeda A, Conrad EH (2015) Semiconducting graphene from highly ordered substrate interactions. Phys Rev Lett 115:136802
Anithaa VS, Shankar R, Vijayakumar S (2017) DFT-based investigation on adsorption of methane on pristine and defected graphene. Struct Chem 28:1935–1952
Li M, Nykypanchuk D, Cotlet M (2018) Improving the responsivity of hybrid graphene–conductive polymer photodetectors via nanowire self-assembly. ACS Photon 5:4296–4302
Dosi M, Lau I, Zhuang Y, Simakov DSA, Fowler MW, Pope MA (2019) Ultrasensitive electrochemical methane sensors based on solid polymer electrolyte-infused laser-induced graphene. ACS Appl Mater Interfaces 11:6166–6173
Erlekam U, Frankowski M, Meijer G, von Helden G (2006) An experimental value for the B1u C–H stretch mode in benzene. J Chem Phys 124:171101
Hashimoto S, Fujimori T, Tanaka H, Urita K, Ohba T, Kanoh H, Itoh T, Asai M, Sakamoto H, Niimura S, Endo M, Rodriguez-Reinoso F, Kaneko K (2011) Anomaly of CH4 molecular assembly confined in single-wall carbon nanohorn spaces. J Am Chem Soc 133:2022–2024
Dinadayalane TC, Leszczynski J (2009) In the pursuit of small “red shift” of C–H stretching vibrational frequency of C–H⋯π interactions for benzene dimer: how to amend MP2 calculations to reproduce the experimental results. J Chem Phys 130:081101
Zhang G, Wang W, Chen D (2008) Chemical origin of blue and red shifts of C–H stretching vibrations in M+−C2H2 (M=V, Fe, Co, Ni) and M+−C6H6 (M=V, Si, Ni) complexes. Chem Phys 354:225–229
Hermida-Ramón JM, Graña AM (2007) Blue-shifting hydrogen bond in the benzene–benzene and benzene–naphthalene complexes. J Comput Chem 28:540–546
Dinadayalane TC, Paytakov G, Leszczynski J (2013) Computational study on C–H⋯π interactions of acetylene with benzene, 1,3,5-trifluorobenzene and coronene. J Mol Model 19:2855–2864
Hobza P, Špirko V, Selzle HL, Schlag EW (1998) Anti-hydrogen bond in the benzene dimer and other carbon proton donor complexes. J Phys Chem A 102:2501–2504
Wang W, Pitoňák M, Hobza P (2007) C-H stretching vibrational shift of benzene dimer: consistency of experiment and calculation. ChemPhysChem 8:2107–2111
Funding
TD recognizes the National Science Foundation (NSF) for the grants through HBCU-UP RIA (Grant Number: 1601071), HBCU-UP TIP (Grant Number: 1623287), and RISE (Grant Number: 1924204). Graduate Studies Office at Clark Atlanta University is thanked for the graduate student support to JL through Title III program. DD acknowledges Saudi Arabian Cultural Mission (SACM) for the scholarship. Extreme Science and Engineering Discovery Environment (XSEDE) is acknowledged for the computational resources (resource allocation grant DMR 160170).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The top and/or side views along with BSSE corrected and uncorrected binding energies, and M06-2X/6-31G(d) level optimized Cartesian coordinates of all molecules and/or complexes considered, selected C-H stretching frequencies and their intensities, data for methane dimer complex are provided. The Supporting Information is available free of charge at https://www.springer.com/ (PDF 7409 kb)
Rights and permissions
About this article
Cite this article
Lazare, J., Daggag, D. & Dinadayalane, T. DFT study on binding of single and double methane with aromatic hydrocarbons and graphene: stabilizing CH…HC interactions between two methane molecules. Struct Chem 32, 591–605 (2021). https://doi.org/10.1007/s11224-020-01657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01657-y