Abstract
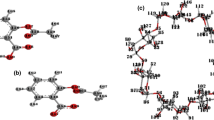
The long-range and dispersion corrected density functional theory (DFT + Disp), and Møller–Plesset second-order perturbation theory (MP2) were used for describing the intermolecular interactions between hydrogen bond driven self-assembly of 2(5-CN-res) … 2(4,4′-bpe) and 2(4,6-diCl-res) … 2(4,4′-bpe) cocrystals [where 5-CN-res = 5-cyanoresorcinol, 4,6-diCl-res = 4,6-dichlororesorcinol, and 4,4′-bpe = trans-1,2-bis(4-pyridyl)ethylene], before and after [2 + 2] dimerization to 2(5-CN-res) … (4,4′-tpcb) and 2(4,6-diCl-res) … (4,4′-tpcb), respectively [where 4,4′-tpcb = 1,2,3,4-tetra(4-pyridyl)cyclobutane]. The nature and strength of intermolecular forces were studied using the absolutely localized molecular orbitals energy decomposition analysis, and the plot of reduced density gradient versus the electron density multiplied by the sign of the second Hessian eigenvalue [sign(λ2)ρ]. The results show that the interaction of 2(4,4′-bpe) is basically dispersive nature, while all of the electrostatic, dispersion, polarization and charge-transfer interactions are largely contributed to the interaction energy of 2(4,4′-bpe) with 5-CN-res and 4,6-diCl-res molecules. The total interaction energy of complexes before dimerization is greater than that after dimerization. Since the contribution of polarization and charge-transfer interactions after dimerization are nearly unchanged, the main difference in the interaction energy of complexes is due to the weaker contribution of van der Waals and electrostatic forces in the products.



Similar content being viewed by others
References
Kaplan IG (2006) Intermolecular interactions: physical picture, computational methods and model potentials. Wiley and Sons, Chichester
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, New York
Huang Z, Yu L, Dai Y (2010) Combined DFT with NBO and QTAIM studies on the hydrogen bonds in (CH3OH)n (n = 2–8) clusters. Struct Chem 21:565–572
Huang Z, Yu L, Dai Y, Wang H (2011) Hydrogen bonding interactions between N, N-dimethylformamide and cysteine: DFT studies of structures, properties, and topologies. Struct Chem 22:57–65
Wang H, Huang Z, Shen T, Guo L (2012) Theoretical study on the hydrogen bonding interactions in 1:1 supermolecular complexes of noradrenaline with water. Struct Chem 23:1163–1172
Kaur D, Kohli R (2012) Hydrogen bonding ability and acid-base behavior of formylphosphinous acid: an isostere of formohydroxamic acid. Struct Chem 23:1879–1890
Rybarczyk-Pirek AJ (2012) Co-crystal/salt crystal structure disorder of trichloroacetic acid-N-methylurea complex with double system of homo- and heteronuclear O-H…O/N–H…O hydrogen bonds: X-ray investigation, ab initio and DFT studies. Struct Chem 23:1739–1749
Kharat B, Deshmukh V, Chaudhari A (2012) Hydrogen-bonding interactions in acetonitrile oligomers using density functional theory method. Struct Chem 23:637–644
Qiu ZM, Cai HZ, Xi HP, Xia YM, Wang HJ (2011) MP2 study on the hydrogen-bonding interaction between 5-hydroxymethyl-uracil and DNA bases: A, C, G, T. Struct Chem 22:509–516
Novakovskaya YV (2012) Conjugation in hydrogen-bonded systems. Struct Chem 23:1253–1266
Oliveira BG, Araujo RCMU, Silva JJ, Ramos MN (2010) A theoretical study of three and four proton donors on linear HX…BeH2…HX and bifurcate BeH2…2HX trimolecular dihydrogen-bonded complexes with X = CN and NC. Struct Chem 21:221–228
Zhang X, Zeng Y, Li X, Meng L, Zheng S (2011) A computational study on the nature of the halogen bond between sulfides and dihalogen molecules. Struct Chem 22:567–576
Li P, Zhai Y, Wang W, Ma Z, Bi S, Sun H (2011) Explorations of the nature of the coupling interactions between vitamin C and methylglyoxal: a DFT study. Struct Chem 22:783–793
Ilnicka A, Sadlej J (2012) Inverse hydrogen bond: theoretical investigation on the nature of interaction and spectroscopic properties. Struct Chem 23:1323–1332
Pakhira S, Sahu C, Sen K, Das AK (2012) Dispersion corrected double high-hybrid and gradient-corrected density functional theory study of light cation-dihydrogen (M+-H2, where M = Li, Na, B and Al) van der Waals complexes. Struct Chem doi: 10.1007/s11224-012-0107-y
Morokuma KJ (1971) Molecular orbital studies of hydrogen bonds. III. C-O…H-O hydrogen bond in H2CO…H2O and H2CO…2H2O. J Chem Phys 55:1236
Kitaura K, Morokuma K (1976) A new energy decomposition scheme for molecular interactions within the Hartree–Fock approximation. Int J Quantum Chem 10:325–340
Umeyama H, Morokuma K (1977) The origin of hydrogen bonding. An energy decomposition study. J Am Chem Soc 99:1316–1332
Bagus PS, Hermann K, Bauschlicher CW (1984) A new analysis of charge transfer and polarization for ligand–metal bonding: Model studies of Al4CO and Al4NH3. J Chem Phys 80:4378
Stevens WJ, Fink WH (1987) Frozen fragment reduced variational space analysis of hydrogen bonding interactions. Application to the water dimmer. Chem Phys Lett 139:15–22
Sokalski WA, Roszak S, Pecul K (1988) An efficient procedure for decomposition of the SCF interaction energy into components with reduced basis set dependence. Chem Phys Lett 153:153–159
Ziegler T, Rauk A (1979) A theoretical study of the ethylene-metal bond in complexes between copper(1 +), silver(1 +), gold(1 +), platinum(0) or platinum(2 +) and ethylene, based on the Hartree–Fock–Slater transition-state method. Inorg Chem 18:1558–1565
Ziegler T, Rauk A (1979) Carbon monoxide, carbon monosulfide, molecular nitrogen, phosphorus trifluoride, and methyl isocyanide as.sigma. donors and.pi. acceptors. a theoretical study by the Hartree–Fock–Slater transition-state method. Inorg Chem 18:1755–1759
Chen W, Gordon MS (1996) Energy decomposition analyses for many-body interaction and applications to water complexes. J Phys Chem 100:14316–14328
Glendening ED (1996) Natural energy decomposition analysis: explicit evolution of electrostatic and polarization effects with application to aqueous clusters of alkali metal cations and neutrals. J Am Chem Soc 118:2473–2482
Mo Y, Gao J, Peyerimhoff SD (2000) Energy decomposition analysis of intermolecular interactions using a block-localized wave function approach. J Chem Phys 112:5530–5538
te Velde G, Bickelhaupt FM, Baerends EJ, van Gisbergen SJA, Fonseca Guerra C, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Lein M, Frenking G (2005) In: Dykstra CE, Frenking G, Kim KS, Scuseria GE (eds) Theory and applications of computational chemistry: the first forty years. Elsevier, Amsterdam
Kovacs A, Esterhuysen C, Frenking G (2005) The nature of the chemical bond revisited: an energy-partitioning analysis of nonpolar bonds. Chem Eur J 11:1813–1825
von Hopffgarten M, Frenking G (2012) Energy decomposition analysis. WIREs Comput Mol Sci 2:43–62
Glendening ED (2005) Natural energy decomposition analysis: extension to density functional methods and analysis of cooperative effects in water clusters. J Phys Chem A 109:11936–11940
Cembran A, Song L, Mo Y, Gao J (2009) Block-localized density functional theory (BLDFT), diabatic coupling, and their use in valence bond theory for representing reactive potential energy surfaces. J Chem Theory Comput 5:2702–2716
Mo Y, Bao P, Gao J (2011) Energy decomposition analysis based on a block-localized wave function and multistate density functional theory. Phys Chem Chem Phys 13:6760–6775
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:014102
Khaliullin RZ, Cobar EA, Lochan RC, Bell AT, Head-Gordon M (2007) Unravelling the origin of intermolecular interactions using absolutely localized molecular orbitals. J Phys Chem A 111:8753–8765
Khaliullin RZ, Bell AT, Head-Gordon M (2009) Electron donation in the water–water hydrogen bond. Chem Eur J 15:851–855
Cobar EA, Horn PR, Bergman RG, Head-Gordon M (2012) Examination of the hydrogen-bonding networks in small water clusters (n = 2–5, 13, 17) using absolutely localized molecular orbital energy decomposition analysis. Phys Chem Chem Phys 14:15328–15339
Khaliullin RZ, Bell AT, Head-Gordon M (2008) Analysis of charge transfer effects in molecular complexes based on absolutely localized molecular orbitals. J Chem Phys 128:184112
Sokolov AN, Bucar D-K, Baltrusaitis J, Gu SX, MacGillivray LR (2010) Supramolecular catalysis in the organic solid state through dry grinding. Angew Chem Int Ed 49:4273–4277
Karunatilaka C, Bucar D-K, Ditzler LR, Friscic T, Swenson DC, MacGillivray LR, Tivanski AV (2011) Softening and hardening of macro- and nano-sized organic cocrystals in a single-crystal transformation. Angew Chem 123:8801–8805
Delori A, Friscic T, Jones W (2012) The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. Cryst Eng Comm 14:2350–2362
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Grimme S, Anthony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Shao Y, Fusti-Molnar L, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O’Neill DP, DiStasio RA Jr, Lochan RC, Wang T, Beran GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khaliullin RZ, Klunzinger P, Lee AM, Lee MS, Liang W, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee YM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock HL III, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF III, Kong J, Krylov AI, Gill PMW, Head-Gordon M (2006) Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys 8:3172–3191
Shao Y, Fusti-Molnar L, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, Gilbert ATB, Slipchenko LV, Levchenko SV, O’Neill DP, DiStasio RA Jr, Lochan RC, Wang T, Beran GJO, Besley NA, Herbert JM, Lin CY, Van Voorhis T, Chien SH, Sodt A, Steele RP, Rassolov VA, Maslen PE, Korambath PP, Adamson RD, Austin B, Baker J, Byrd EFC, Dachsel H, Doerksen RJ, Dreuw A, Dunietz BD, Dutoi AD, Furlani TR, Gwaltney SR, Heyden A, Hirata S, Hsu C-P, Kedziora G, Khaliullin RZ, Klunzinger P, Lee AM, Lee MS, Liang W, Lotan I, Nair N, Peters B, Proynov EI, Pieniazek PA, Rhee YM, Ritchie J, Rosta E, Sherrill CD, Simmonett AC, Subotnik JE, Woodcock HL III, Zhang W, Bell AT, Chakraborty AK, Chipman DM, Keil FJ, Warshel A, Hehre WJ, Schaefer HF III, Kong J, Krylov AI, Gill PMW, Gan Z, Zhao Y, Schultz NE, Truhlar D, Epifanovsky E, Oana M, Baer R, Brooks BR, Casanova D, Chai J-D, Cheng C-L, Cramer C, Crittenden D, Ghysels A, Hawkins G, Hohenstein EG, Kelley C, Kurlancheek W, Liotard D, Livshits E, Manohar P, Marenich A, Neuhauser D, Olson R, Rohrdanz MA, Thanthiriwatte KS, Thom AJW, Vanovschi V, Williams CF, Wu Q, You Z-Q, Head-Gordon M (2011) Q-Chem, Version 4.0. Q-Chem, Inc, Pittsburgh PA
Kalugina YN, Cherepanov VN, Buldakov MA, Zvereva-Loete N, Boudon V (2012) Theoretical investigation of the ethylene dimer: interaction energy and dipole moment. J Comput Chem 33:319–330
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing non-covalent interactions. J Am Chem Soc 132:6498–6506
Acknowledgments
We would like to thank the Isfahan University of Technology’s research council for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamani, M., Dabbagh, H.A. Quantitative analysis of intermolecular forces for hydrogen bond driven self-assembly of resorcinol and bis(pyridine) substituted ethylene cocrystals, before and after [2 + 2] dimerization. Struct Chem 24, 1597–1605 (2013). https://doi.org/10.1007/s11224-012-0197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0197-6