Abstract
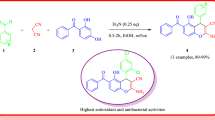
Several derivatives of 4H-chromene and chromeno[2,3-b]pyridine were efficiently prepared under microwave irradiation in a one-pot reaction, and their anti-inflammatory activities were evaluated. Six synthetic products (1b, 1c, 1h, 2d, 2j, and 2l) exhibited more powerfully inhibited the production of tumor necrosis factor-α-induced nitric oxide (NO) than quercetin and exhibited comparable cell viability in both human and porcine chondrocytes. In particular, 2d at dosages of 10 and 20 mg/kg had a very potent anti-inflammatory effect by suppressing the formation of carrageenan-induced rat paw edema and prostaglandin E2. The results herein suggest that these compounds may have potential as structural templates in the design and development of new anti-inflammatory drugs.





Similar content being viewed by others
References
D.A. Walsh, D.F. McWilliams, Nat. Rev. Rheumatol. 10, 581–592 (2014)
H.B. Qin, B. Xu, J.J. Mei, D. Li, J.J. Liu, D.Y. Zhao, F. Liu, Inflammation 35, 1595–1599 (2012)
C.A. Akdis, Nat. Med. 18, 736–749 (2012)
M.Y. Donath, Nat. Rev. Drug Discov. 13, 465–476 (2014)
M.E. Rosenfeld, Curr. Opin. Pharmacol. 13, 154–160 (2013)
A. Mantovani, P. Allavena, A. Sica, F. Balkwill, Nature 454, 436–444 (2008)
L.J. Crofford, Arthritis Res. Ther. 15, S2 (2013)
M. Abdel-Aziz, G. El-Din, A.A. Abuo-Rahma, E.A. Beshr, T.F. Ali, Bioorg. Med. Chem. 21, 3839–3849 (2013)
G.F. Dai, J. Zhao, Z.W. Jiang, L.P. Zhu, H.W. Xu, W.Y. Ma, X.R. Chen, R.J. Dong, W.Y. Li, H.M. Liu, Int. Immunopharmacol. 11, 2144–2149 (2011)
J. Yang, S. Li, C. Xie, H. Ye, H. Tang, L. Chen, A. Peng, J. Ethnopharmacol. 147, 442–446 (2013)
A. Adatia, K.D. Rainsford, W.F. Kean, J. Pharm. Pharmacol. 64, 626–636 (2012)
C. Musumba, D.M. Pritchard, M. Pirmohamed, Aliment. Pharmacol. Ther. 30, 517–531 (2009)
C.P. Cannon, P.J. Cannon, Science 336, 1386–1387 (2012)
H. Bao, Y. Ge, S. Zhuang, L.D. Dworkin, Z. Liu, R. Gong, Kidney Int. 81, 662–673 (2012)
G.P. Aithal, Nat. Rev. Rheumatol. 7, 139–150 (2011)
A. Nohara, T. Ishiguro, K. Ukawa, H. Sugihara, Y. Maki, Y. Sanno, J. Med. Chem. 28, 559–568 (1985)
M.J. Martin, C. La-Casa, C. Alarcon-de-la-Lastra, J. Cabeza, I. Villegas, V. Motilva, Z. Naturforsch. C. 53, 82–88 (1998)
W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, C. Crogan-Grundy, D. Labreque, M. Bubenick, G. Attardo, R. Denis, S. Lamothe, H. Gourdeau, B. Tseng, S. Kasibhatla, S.X. Cai, J. Med. Chem. 51, 417–423 (2008)
A.M. Shestopalov, Y.M. Litvinov, L.A. Rodinovskaya, O.R. Malyshev, M.N. Semenova, V.V. Semenov, ACS Combust. Sci. 14, 484–490 (2012)
D. Panda, J.P. Singh, L. Wilson, J. Biol. Chem. 272, 7681–7687 (1997)
R.R. Raju, S.K. Mohan, S.J. Reddy, J. Sci. Ind. Res. 62, 334–338 (2003)
D.D. Haveliwala, N.R. Kamdar, P.T. Mistry, S.K. Patel, Helv. Chim. Acta 96, 897–905 (2013)
N.R. Kamdar, D.D. Haveliwala, P.T. Mistry, S.K. Patel, Eur. J. Med. Chem. 45, 5056–5063 (2010)
X.P. Lee, T. Kumazawa, C. Hasegawa, T. Arinobu, A. Kato, H. Seno, K. Sato, Forensic Toxicol. 28, 96–104 (2010)
I. Akyol-Salman, D. Lece-Sertoz, O. Baykal, J. Ocul. Pharmacol. Ther. 23, 280–283 (2007)
M. Ahuja, A.S. Dhake, S.K. Sharma, D.K. Majumdar, AAPS J. 10, 229–241 (2008)
W. Kemnitzer, J. Drewe, S.C. Jiang, H. Zhang, Y. Wang, J.H. Zhao, S.J. Jia, J. Herich, D. Labreque, R. Storer, K. Meerovitch, D. Bouffard, R. Rej, R. Denis, C. Blais, S. Lamothe, G. Attardo, H. Gourdeau, B. Tseng, S. Kasibhatla, S.X. Cai, J. Med. Chem. 47, 6299–6310 (2004)
A. Olyaei, M. Vaziri, R. Razeghi, Tetrahedron Lett. 54, 1963–1966 (2013)
A.C. Shekhar, A.R. Kumar, G. Sathaiah, K. Raju, P.S. Rao, M. Sridhar, B. Narsaiah, P.V.S.S. Srinivas, B. Sridhar, Helv. Chim. Acta 95, 502–508 (2012)
S.B. Cheng, Master Thesis, National Defense Medical Center (2003)
C.A. Winter, E.A. Risley, G.W. Nuss, Proc. Soc. Exp. Biol. Med. 111, 544–547 (1962)
W.H. Huang, C.L. Yang, A.R. Lee, H.F. Chiu, Chem. Pharm. Bull. 51, 313–314 (2003)
K.R. Desale, K.P. Nandre, S.L. Patil, Org. Commun. 5, 179–185 (2012)
F.C. Liu, L.F. Hung, W.L. Wu, D.M. Chang, C.Y. Huang, J.H. Lai, L.J. Ho, Arthritis Res. Ther. 12, R167 (2010)
J. Guay, K. Bateman, R. Gordon, J. Mancini, D. Riendeau, J. Biol. Chem. 279, 24866–24872 (2004)
Acknowledgments
We gratefully acknowledge the research grant, NSC 103-2320-B-016-009, supported from the National Science Council of the Republic of China. The authors would like to thank Professor Ted Knoy for his editorial assistance.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standard
This study was performed in accordance with the criteria of the National Academy of Sciences, and approved by the Institutional Animal Care and Use Committee of National Defense Medical Center, Taipei, Taiwan (Approval Number: IACUC-13-178). All patients enrolled with informed consent in this study were purposively sampled under Tri-Service General Hospital Institution Review Board approval (Approval Number: 1-102-05-091).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, ST., Huang, WH., Huang, CK. et al. Synthesis and anti-inflammatory activities of 4H-chromene and chromeno[2,3-b]pyridine derivatives. Res Chem Intermed 42, 1195–1215 (2016). https://doi.org/10.1007/s11164-015-2081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2081-7