Abstract
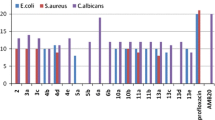
A series of tetrazolomethylbenzo[d][1,2,3]triazole derivatives (2–14) have been synthesized and evaluated as antimicrobial agents from 1H-benzo[d][1,2,3]triazole (1) as starting material. The reaction of benzotriazole 1 with chloroacetonitrile afforded 2-(1H-benzo[d][1,2,3]-triazol-1-yl)acetonitrile 2, which was reacted with sodium azide to give tetrazole derivative 3. Esterification of benzotriazole 1 with ethyl bromoacetate in the presence of anhydrous potassium carbonate afforded ester 4, which was treated with hydrazine hydrate to afford the corresponding hydrazide 5. Reaction of 3 with 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide afforded the nitro-glycoside derivative 6, which was deacetylated using methanolic ammonia to deprotected nitroglycoside 7. The hydrazide 5 was reacted with 4,5,6,7-tetrachlorophthalic anhydride or 1,2,4,5-benzenetetracarboxylic dianhydride in refluxing glacial acetic acid to give the corresponding imides 8 and 9, respectively. Also, the hydrazide 5 was reacted with carbon disulphide in ethanol to give potassium salt 10, which was reacted with hydrazine hydrate to afford aminotriazole derivative 11. The latter compound was reacted with carbon disulphide to afford thiadiazole derivative 12, which was treated with 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide to give the thioglycoside derivative 13. Deacetylation of the thioglycoside 13 using methanolic ammonia solution at room temperature afforded the deprotected thioglycoside 14. The antimicrobial screening of some synthesized compounds showed that many of these compounds have good antimicrobial activities comparable to streptomycin and fusidic acid as reference drugs.




Similar content being viewed by others
References
N. Klose, K. Niedbolla, K. Schwartz, I. Bottcher, Arch. Pharm. 316, 941 (1983)
R.K. Satsangi, S.M. Zaidi, V.C. Misra, Pharmazie 38, 341 (1983)
R. Pignatello, S. Mazzone, A.M. Panico, G. Mazzone, G. Penissi, R. Castano, M. Matera, G. Blandino, Eur. J. Med. Chem. 26, 929 (1991)
D. Hadjipavlou-Litina, A. Geronikaki, E. Sotiropoulou, Res. Commun. Chem. Pathol. Pharmacol. 79, 355 (1993)
A. Geronikaki, D. Hadjipavlou-Litina, Pharmazie 48, 948 (1993)
E.S. Lazer, H.C. Wong, G. Possanza, A.G. Graham, P.R. Farina, J. Med. Chem. 32, 100 (1989)
J.G. Michael, M.L. Rachel, L.M. Susan, H.B. John, L.B. Milton, Bioorg. Med. Chem. 12, 1029–1036 (2004)
A. Geronikaki, D. Hadjipavlou-Litina, C. Chatziopoulos, G. Soloupis, Molecules 8, 472 (2003)
K.Y. Jung, S.K. Kim, Z.G. Gao, S.G. Ariel, M. Neli, A.J. Denneth, Y.C. Kim, Bioorg. Med. Chem. 12, 613 (2004)
S. Tehranchian, T. Akbarzadeh, M.R. Fazeli, H. Jamalfar, A. Shafiee, Bioorg. Med. Chem. Lett. 15, 1023 (2005)
G. Turan-Zitouni, Z.A. Kaplancikli, M.T. Yildiz, P. Chevallet, D. Kaya, Eur. J. Med. Chem. 40, 607 (2005)
V. Padmavathi, G. Sudhakar, A. Reddy, P. Padmaja, Kondaiah, S. Ali, Eur. J. Med. Chem. 44, 2106 (2009)
H. Bayrak, A. Demirbas, S.A. Karaoglu, N. Demirbas, Eur. J. Med. Chem. 44, 1057 (2009)
M. Ashok, B.S. Holla, B. Boojary, Eur. J. Med. Chem. 42, 1095 (2007)
M.S. Karthikeyan, D.J. Prasad, B. Boojary, K.S. Bhat, B.S. Holla, N.S. Kumari, Bioorg. Med. Chem. 14, 7482 (2006)
B. Tozkoparan, E. Küpeli, E. Yeşilada, M. Ertan, Bioorg. Med. Chem. 15, 1808 (2007)
L. Labanauskas, E. Udrenaite, P. Gaidelis, A. Brukštus, II Farmaco 59, 255 (2004)
L. Navidpour, H. Shafaroodi, K. Abdi, M. Amini, M.H. Ghahremani, A.R. Dehpour, A. Shafiee, Bioorg. Med. Chem. 14, 2507 (2006)
J.R. Maxwell, D.A. Wasdahl, A.C. Wolfson, V.I. Stenberg, J. Med. Chem. 27, 1565 (1984)
A.E. Amr, M. Abo-Ghalia, M.M. Abdalah, Z. Naturforsch. 61b, 1335 (2006)
A.E. Amr, A.M. Mohamed, A.A. Ibrahim, Z. Naturforsch. 58b, 861 (2003)
A.E. Amr, A.M. Mohamed, S.F. Mohamed, N.A. Abdel-Hafez, A.G. Hammam, Bioorg. Med. Chem. 14, 5481 (2006)
A.E. Amr, N.M. Sabrry, M.M. Abdalla, B.F. Abdel-Wahab, Eur. J. Med. Chem. 44, 725 (2009)
I.M. Fakhr, A.E. Amr, N.M. Sabry, M.M. Abdalah, Arch. Pharm. Chem. Life Sci. 341, 174 (2008)
S.F. Mohamed, E.M. Flefel, A.E. Amr, D.N. Abd El-Shafy, Eur. J. Med. Chem. 45, 1494 (2010)
A.E. Amr, K.A. Ali, M.M. Abdalla, Eur. J. Med. Chem. 44, 901–907 (2009)
G.L. Furtada, A.A. Medeiros, J. Clin. Microbiol. 12, 550 (1980)
R.N. Jones, C.H. Ballow, D.J. Biedenbach, Diagn. Microbiol. Infect. Dis. 40, 59 (2001)
M.M. Ramla, M.A. Omar, H. Tokuda, H.I. El-Diwani, Bioorg. Med. Chem. 15, 6489 (2007)
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-0172.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, O.M., Amr, A.EG.E. & Mostafa, E.E. Synthesis and antimicrobial of some new substituted tetrazolomethylbenzo[d]-[1,2,3]triazole derivatives using 1H-benzo[d][1,2,3]triazole as starting material. Res Chem Intermed 40, 1545–1556 (2014). https://doi.org/10.1007/s11164-013-1059-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1059-6