Abstract
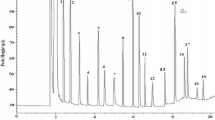
The baobab seed and pulp were analyzed for proximate composition, mineral content, and amino acid composition. The seed oil and protein were evaluated for their fatty acid profile and protein solubility. The seed was found to be a good source of energy, protein, and fat. Both the kernel and the pulp contain substantial quantities of calcium, potassium, and magnesium. Amino acid analyses revealed high glutamic and aspartic acid contents and the sulfur-containing amino acids as being the most limited amino acid. The fatty acid profile showed that oleic and linoleic were the major unsaturated fatty acids, whereas palmitic was the major saturated acid. Of the several solvents tested to solubilize the seed protein, 0.1 M NaOH was found to be the most effective. The protein was more soluble at alkaline than acidic pH, with the lowest solubility at pH 4.0.
Similar content being viewed by others
References
Yazzie D, Van der Jaget DJ, Pastuszen A, Okolo A, Glwu RH (1994) The amino acid and mineral content of baobab (Adansoina digitata L.) Leaves. J Food Comp Anal 7: 189–193.
Obizoba TC, Amaechi NA (1983) The effect of processing methods on the chemical composition of baobab (Adansonia digitata) pulp and seed. Ecol Food Nutr 29: 199–205.
Addy EO, Eteshola E (1984) Nutritive value of mixture of tigernut tubers (Cyperus esculentus L.) and baobab seeds (Adansonia digitata L.). J Sci Food Agric 35: 437–440.
AOAC (1990) Official Methods of Analysis, 15th ed. Washington, DC: Association of Official Analytical Chemists.
Spackman DH, Stein WH, Moore S (1958) Automatic apparatus for use in the chromatography of amino acids. Anal Chem 30: 1190–1195.
Kakade ML, Simon N, Liener IE (1969) An evaluation of natural vs. synthetic substrate for measuring anititryptic activity of soybean samples. Cereal Chem 46: 518–526.
Mohamed A, Perera PJ, Hafez YS (1986) New chromaphore for phytic acid determination. Cereal Chem 63: 475–478.
Price ML, Scoyoc VS, Butter LG (1978) A ctirical evaluation of reaction as assay for tannin in sorghum grain. J Agric Food Chem 26: 1214–1218.
Metcalf LC, Schmitz AA, Pleca JR Jr (1966) Rapid preparation of fatty acid ester from lipid for gas chromatography analysis. Anal Chem 38: 514–515.
Sathe SK (1994) Solubilization and electrophoretic characterization of Cashew nut (Anacardium occidentale) proteins. Food Chem 51: 319–324.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193: 265–277.
Nour AA, Magboul BI, Kheiri NH (1980) Chemical composition of baobab (Adnasonia digitata L.). Trop Sci 22: 383–388.
Lockett TC, Calvert CC, Grivetti EL (2000) Energy and micronutrients composition of dietary and medical wild plant consumed during drought, study of rural Fulani Northeastern Nigeria. Int J Food Sci Nut 51: 57–72.
Glew HR, Van der Jaget JD, Lockette TC, Grivetti EL, Smith CG, Pastuszyn A, Milsom M (1997) Amino acid, fatty acid and mineral composition of 24 indigenous plants of Burkino Faso. J Food Comp Anal 10: 205–217.
Ralaimanerivo A, Gaydou EM, Bianchini JB (1982) Fatty acid composition of seed from six Adansonia species with particular reference to cyclopropane and cydopropene acid. Lipids 17: 1–10.
Prentice A, Laskey MA, Shaw J, Hudson GJ, Day KC, Jarjou MA, Dibba B, Paul AA (1993) The calcium and phosphorus intakes of rural Gambian woman during pregnancy and lactation. Br J Nutr 69: 885–896.
Idouraine A, Weber CW, Sathe SK, Kohlhepp P (1992) Antinutritional factors in protein fraction of tepary bean. Food Chem 45: 37–39.
Anantharaman K, Carpenter KJ (1969) Effect of heat processing on the nutritional value of ground nut products 1. Protein quality of ground nut cotyledons for rats. J Sci Food Agric 20: 703–708.
Mir Z, Hill DC (1979) Nutritional value of peanut meal from Ontario grown peanut compared with a meal from U.S. grown peanut and soybean meat. J Can Inst Food Sci Technol 12: 56–60.
Ologhobo DA, Fetuga BL (1983) Trypsin inhibitor activity in some lima bean varieties as affected by different processing method. Nutr Rep Int 27: 41–48.
Reddy NR, Pierson MD, Sathe SK, Salunkhe DK (1989) Phytate in Cereal and Legumes. Boca Raton, FL: Press CRC, P 154.
Sathe SK, Salunkhe DK (1981a) Investigation of winged bean [Psophocarpus tetragonalobus (L) DCJ protein and antinutritional factors]. J Food Sci 46: 1309–1393.
Souza PA (1987) Avalico bromatalogica, nutricional e technologica de algumas leguminosas tropicais. Sao Paulo: FCF/USP.
Reddy NR, Pierson MD, Sathe SK, Salunkhe DK (1985) Dry bean tannin: a review of nutritional implication. J Am Oil Chem Soc 62: 451–549.
Muindi PJ, Thomke S (1981) The nutritive value for rat of high and low tannin sorghum treated with Magadi sod. J Sci Food Agric 32: 139–145.
Proll J, Petzk KJ, Ezeagu IE, Metges CC (1998) Low nutritional quality of unconventional tropical crops seed in rats. J Nutr 128: 2014–2022.
Okoye WI, Kazaure I, Egesi GV (1980) A preliminary investigation of baobab (Adansonia digitata L.) as a potential source of oilseed. Annual Report Nigerian Stored Product Research Institute 1977–1978: pp.73–75.
Gaydou EM, Bianchini JP, Ralaimanarivo A (1979) African baobab oil: Adansonia digital L. Fatty acid and sterol compositon. Revue Francaise des Crops Gras 26: 447–448.
Sathe SK, Salunkhe DK (1981) Solubilization and electrophoretic characterization of the great Northern pea (Phaseolus vulgaris L.) J Food Sci 46, 82–87.
Basha SMM, Cherry JP (1976) Composition, solubility and gel electrophoretic properties of protein isolated florunner (Archis hypogaea) peanut seed. J Agric Food Chem 24: 359–365.
Shokarri HE, Esen A (1988) Composition, solubility and electrophoretic pattern proteins isolated from kerman pistachio nuts (Pistacia vera L.). J Agric Food Chem 36: 425–429.
Idouraine A, Yensen SB, Weber CW (1991) Tepary bean flour, albumin and globulin fractions functional properties compared with soy protein isolate. J Food Sci 56: 1316–1319.
Sathe SK, Salunkhe DK (1981) Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: Emulsion, foaming, viscosity and gelation properties. J Food Sci 46: 71–87.
Sosulski FW, McCurdy AR (1987) Functionality of flours, protein fractions and isolate from field pea and faba bean. J Food Sci 52: 1010–1114.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
OSMAN, M.A. Chemical and Nutrient Analysis of Baobab (Adansonia digitata) Fruit and Seed Protein Solubility. Plant Foods Hum Nutr 59, 29–33 (2004). https://doi.org/10.1007/s11130-004-0034-1
Issue Date:
DOI: https://doi.org/10.1007/s11130-004-0034-1