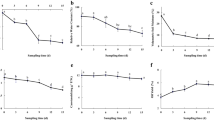
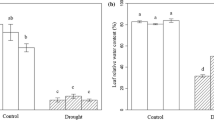
Abstract
Metabolic responses are important for plant adaptation to abiotic stress. To investigate the responses of Phlox subulata L. to drought stress, we analyzed its physiological and metabolic changes using gas chromatography-mass spectrometer. Based on the physiological indices, P. subulata L. has tolerance to drought to some degree. Our results showed that there were a total of 30 key metabolites induced by drought stress, including amino acids, organic acids, sugars and sugar alcohols, nucleic acid and its derivatives, and other organic compounds. The glutamic acid-mediated proline biosynthesis pathway is continuously upregulated under drought stress, which could regulate osmotic pressure and maintain intracellular environmental stability. More secondary metabolites are used to increase glycolysis and tricarboxylic acid cycle, to accelerate energy production and to enhance the glutamic acid-mediated proline biosynthesis pathway, which are necessary to increase osmotic regulation. Prolonged drought stress induced progressive accumulation of compatible osmolytes, such as proline and inositol, sugars, and amino acids. Therefore, drought caused systemic alterations in metabolic networks involving transamination, TCA cycle, gluconeogenesis/glycolysis, glutamate-mediated proline biosynthesis, shikimate-mediated secondary metabolisms, and the metabolism of pyrimidine. These data suggest that plants may utilize these physiological and metabolomic adjustments as adaptive responses in the early stages of drought stress. These results deepen our understanding of the mechanisms involved in P. subulata L. drought tolerance, which will help improve the understanding of drought’s effects on plant systems.






Similar content being viewed by others
References
Agarwal PK, Gupta K, Lopato S, Agarwal P (2017) Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J Exp Bot 68:2135–2148
Bowne J, Bacic A, Tester M, Roessner U (2018) Abiotic stress and metabolomics. Ann Plant Rev online:61–85
Bozukov V, Tsenov B, Valchev V, Georgiev V, Tsoneva S (2012) Comptes rendus de l’Academie bulgare des Sciences. Cr Acad Bulg Sci 65:985–990
Calvo-Polanco M, Ruiz-Lozano JM, Azcón R, Molina S, Beuzon CR, García JL, Cantos M, Aroca R (2019) Phenotypic and molecular traits determine the tolerance of olive trees to drought stress. Plant Physiol Biochem 139:521–527
Correia B, Hancock RD, Amaral J, Gomez-Cadenas A, Valledor L, Pinto G (2018) Combined drought and heat activates protective responses in Eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front Plant Sci 9:819
Cui LH, Byun MY, Oh HG, Kim SJ, Lee J, Park H, Lee H, Kim WT (2020) Poaceae type II galactinol synthase 2 from Antarctic flowering plant Deschampsia antarctica and rice improves cold and drought tolerance by accumulation of Raffinose family oligosaccharides in transgenic rice plants. Plant Cell Physiol 61:88–104
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Ding Y, Avramova Z, Fromm M (2011) The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. Plant J 66:735–744
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize leaves. Plant Physiol 114:1031–1037
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Ferguson CJ, Jansen RK (2002) A chloroplast DNA phylogeny of eastern Phlox (Polemoniaceae): implications of congruence and incongruence with the ITS phylogeny. Am J Bot 89:1324–1335
Franco J, Martínez-Sánchez J, Fernández J, Bañón S (2006) Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J Hortic Sci Biotechnol 81:3–17
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17:3470–3488
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Griot C, Vandevelde M, Richard A, Peterhans E (1990) Selective degeneration of oligodendrocytes mediated by reactive oxygen species. Free Radic Res Commun 11:181–193
Guo R et al (2018) Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 10:ply016
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hong Y-H, Meng J, He X-L, Zhang Y-Y, Luan Y-S (2019) Overexpression of MiR482c in tomato induces enhanced susceptibility to late blight. Cells 8:822
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Huang T, Jander G (2017) Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branched-chain amino acids in Arabidopsis thaliana. Planta 246:737–747
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant 84:55–60
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273:1853–1856
Jaleh D (2017) Metabolic adjustments, assimilate partitioning, and alterations in source-sink relations in drought-stressed plants. In: Photoassimilate Distribution Plants and Crops Source-Sink Relationships. Routledge, pp 407–420
Jalil SU, Khan MIR, Ansari MI (2019) Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana. Curr Plant Biol 19:100119
Jiang M, Xu F, Peng M, Huang F, Meng F (2016) Methyl jasmonate regulated diploid and tetraploid black locust (Robinia pseudoacacia L.) tolerance to salt stress. Acta Physiol Plant 38:106
Joshi V, Joung J-G, Fei Z, Jander G (2010) Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids 39:933–947
Kampfenkel K, Vanmontagu M, Inze D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Khan MA, Alghamdi SS, Ammar MH, Sun Q, Teng F, Migdadi HM, Al-Faifi SA (2019a) Transcriptome profiling of faba bean (Vicia faba L.) drought-tolerant variety hassawi-2 under drought stress using RNA sequencing. Electron J Biotechnol 39:15–29
Khan N, Bano A, Rahman MA, Rathinasabapathi B, Babar MA (2019b) UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ 42:115–132
Khanna-Chopra R, Selote DS (2007) Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environ Exp Bot 60:276–283
Kim SY (2006) The role of ABF family bZIP class transcription factors in stress response. Physiol Plant 126:519–527
Kosová K, Vítámvás P, Urban MO, Klíma M, Roy A, Prášil IT (2015) Biological networks underlying abiotic stress tolerance in temperate crops—a proteomic perspective. Int J Mol Sci 16:20913–20942
Kusvuran A, Kusvuran S (2019) Using of microbial fertilizer as biostimulant alleviates damage from drought stress in guar (Cyamopsis Tetragonoloba (L.) Taub.) seedlings. Int Lett Natl Sci 76:147–157
Lambrecht SC (2013) Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). Int J Plant Sci 174:74–84
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6:30338
Luo Q, Peng M, Zhang X, Lei P, Ji X, Chow W, Meng F, Sun G (2017) Comparative mitochondrial proteomic, physiological, biochemical and ultrastructural profiling reveal factors underpinning salt tolerance in tetraploid black locust (Robinia pseudoacacia L.). BMC Genomics 18:648
Ma D, Sun D, Wang C, Ding H, Qin H, Hou J, Huang X, Xie Y, Guo T (2017) Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front Plant Sci 8:860
Meng F, Peng M, Pang H, Huang F (2014) Comparison of photosynthesis and leaf ultrastructure on two black locust (Robinia pseudoacacia L.). Biochem Syst Ecol 55:170–175
Michaletti A, Naghavi MR, Toorchi M, Zolla L, Rinalducci S (2018) Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci Rep 8:5710
Moreno-Galván AE, Cortés-Patiño S, Romero-Perdomo F, Uribe-Vélez D, Bashan Y, Bonilla RR (2020) Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl Soil Ecol 147:103367
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nickel KS, Cunningham B (1969) Improved peroxidase assay method using leuco 2, 3′, 6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis. Anal Biochem 27:292–299
Nordhoff A, Buecheler US, Werner D, Schirmer RH (1993) Folding of the four domains and dimerization are impaired by the Gly446. fwdarw. Glu exchange in human glutathione reductase. Implications for the design of antiparasitic drugs. Biochemistry 32:4060–4066
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Qu Y, Zhou A, Zhang X, Tang H, Liang M, Han H, Zuo Y (2015) De novo transcriptome sequencing of low temperature-treated Phlox subulata and analysis of the genes involved in cold stress. Int J Mol Sci 16:9732–9748
Qu X, Wang H, Chen M, Liao J, Yuan J, Niu G (2019) Drought stress–induced physiological and metabolic changes in leaves of two oil tea cultivars. J Am Soc Hortic Sci 144:439–447
Rahman MA, Akond M, Babar MA, Beecher C, Erickson J, Thomason K, de Jong FA, Mason RE (2017) LC-HRMS based non-targeted metabolomic profiling of wheat (Triticum aestivum L.) under post-anthesis drought stress. Am J Plant Sci 8:3024–3061
Rigui AP, Carvalho V, dos Santos ALW, Morvan-Bertrand A, Prud’homme M-P, de Carvalho MAM, Gaspar M (2019) Fructan and antioxidant metabolisms in plants of Lolium perenne under drought are modulated by exogenous nitric oxide. Plant Physiol Biochem 145:205–215
Ruan C-J, Teixeira da Silva JA (2011) Metabolomics: creating new potentials for unraveling the mechanisms in response to salt and drought stress and for the biotechnological improvement of xero-halophytes. Crit Rev Biotechnol 31:153–169
Sami F, Hayat S (2019) Effect of glucose on the morpho-physiology, photosynthetic efficiency, antioxidant system, and carbohydrate metabolism in Brassica juncea. Protoplasma 256:213–226
Sarwar MB et al (2019) De novo assembly of Agave sisalana transcriptome in response to drought stress provides insight into the tolerance mechanisms. Sci Rep 9-22:396
Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10:296–302
Selvaraj MG, Ishizaki T, Valencia M, Ogawa S, Dedicova B, Ogata T, Yoshiwara K, Maruyama K, Kusano M, Saito K, Takahashi F, Shinozaki K, Nakashima K, Ishitani M (2017) Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol J 15:1465–1477
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Silambarasan S, Logeswari P, Cornejo P, Kannan VR (2019) Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ Sci Pollut Res 26:27647–27659
Singh D, Laxmi A (2015) Transcriptional regulation of drought response: a tortuous network of transcriptional factors. Front Plant Sci 6:895
Singh PK, Srivastava D, Tiwari P, Tiwari M, Verma G, Chakrabarty D (2019) Drought tolerance in plants: molecular mechanism and regulation of signaling molecules. In: Plant Signaling Molecules. Elsevier, pp 105–123
Sofo A, Tuzio AC, Dichio B, Xiloyannis C (2005) Influence of water deficit and rewatering on the components of the ascorbate–glutathione cycle in four interspecific Prunus hybrids. Plant Sci 169:403–412
Springer TL, Gunter SA, Tyrl RJ, Nighswonger PF (2013) Drought and grazing effects on Oklahoma phlox (Polemoniaceae, Phlox oklahomensis). Am J Plant Sci 4:9–13
Stępień P, Kłbus G (2006) Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol Plant 50:610–616
Suguiyama VF, da Silva EA, Meirelles ST, Centeno DDC, Braga MR (2014) Leaf metabolite profile of the Brazilian resurrection plant Barbacenia purpurea Hook.(Velloziaceae) shows two time-dependent responses during desiccation and recovering. Front Plant Sci 5:96
Tardieu F, Simonneau T, Muller B (2018) The physiological basis of drought tolerance in crop plants: a scenario-dependent probabilistic approach. Annu Rev Plant Biol 69:733–759
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Tschaplinski TJ, Abraham PE, Jawdy SS, Gunter LE, Martin MZ, Engle NL, Yang X, Tuskan GA (2019) The nature of the progression of drought stress drives differential metabolomic responses in Populus deltoides. Ann Bot 124:617–626
Wan Q-L, Meng X, Fu X, Chen B, Yang J, Yang H, Zhou Q (2019) Intermediate metabolites of the pyrimidine metabolism pathway extend the lifespan of C. elegans through regulating reproductive signals. Aging (Albany NY) 11:3993
Xu F, Jiang M, Meng F (2017) Short-term effect of elevated CO 2 concentration (0.5%) on mitochondria in diploid and tetraploid black locust (Robinia pseudoacacia L.). Ecol Evol 7:4651–4660
Yang D, Zhang J, Li M, Shi L (2017) Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J Plant Growth Regul 36:460–471
Yu X, Ohtani M, Kusano M, Nishikubo N, Uenoyama M, Umezawa T, Saito K, Shinozaki K, Demura T (2017) Enhancement of abiotic stress tolerance in poplar by overexpression of key Arabidopsis stress response genes, AtSRK2C and AtGolS2. Mol Breed 37:57
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65. https://doi.org/10.1007/s10681-007-9457-8
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10:1904–1914
Zhou R, Yu X, Ottosen CO, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z (2017) Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol 17:24
Zhou A, Sun H, Feng S, Zhou M, Gong S, Wang J, Zhang S (2018) A novel cold-regulated gene from Phlox subulata, PsCor413im1, enhances low temperature tolerance in Arabidopsis. Biochem Biophys Res Commun 495:1688–1694
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Funding
This research was funded by grants from the National Key Research and Development Program of China (No. 2016YFC0500300), special project of basic applied technology research by institutions in Heilongjiang Province (ZNB2020ZR07).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
These results deepen our understanding of the mechanisms involved in P. subulata L. drought tolerance, which will help improve the understanding of drought’s effects on plant systems.
Electronic Supplementary Material
Fig. S1
The morphology (left) and flowers (right) of Phlox subulata L. (PNG 28758 kb)
Fig. S2
The nitro blue tetrazolium (NBT) and diamino benzidine (DAB) staining results of Phlox subulata roots under drought stress A-D: NBT staining to test H2O2; (A) drought stress for 4 days; (B) drought stress for 8 days; (C) drought stress for 12 days; (D) 4 days after rehydration, E-H: DAB staining to test O2−; (E) drought stress for 4 days; (F) drought stress for 8 days; (G) drought stress for 12 days; (H) 4 days after rehydration. DAS: day after drought stress (PNG 3490 kb)
Fig. S3
Changes in GSH/GSSG (A), GSH+GSSG (B), ASA/DHA (C), ASA+DHA (D) of Phlox subulata roots under drought stress. Values represent the means of six separate experiments ± standard deviations (SD). Lowercase letters stand for significant difference at 5% level (PNG 254 kb)
ESM 4
(XLSX 187 kb)
Rights and permissions
About this article
Cite this article
Xiong, Y., Qu, Y., Han, H. et al. Unraveling Physiological and Metabolomic Responses Involved in Phlox subulata L. Tolerance to Drought Stress. Plant Mol Biol Rep 39, 98–111 (2021). https://doi.org/10.1007/s11105-020-01238-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-020-01238-7