Abstract
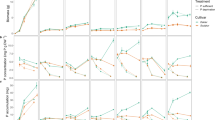
Adequate phosphorus (P) nutrition during early stages is critical for maize growth. Our objective was to evaluate the relative contribution of seed P reserves and exogenous P to maize nutrition during early growth stages. Seedlings were grown with labeled nutrient solution (32P). Seedlings were harvested periodically over the course of the three-week study. Initially, 87% and 77% of the total C and N in seeds were located in the endosperm, whereas 86% of seed P was located in the scutellum as phytate. Up to the 7th day after sowing, 96% of phytate was hydrolyzed. Hydrolyzed forms of P were temporarily stored in the seed before being translocated to growing organs, suggesting that the hydrolysis of phytate was not a limiting step for P supply to seedlings. Significant P uptake by roots was observed from the 5th day after sowing on. Both sources of P supplied roots and leaves, with a slightly higher proportion of P from seed reserves going to leaves rather than to roots. Of total seed P, 60% and 92% was exported towards newly growing seedlings till 7th and 17th days after sowing and ceased to be a significant source of P for growth thereafter. We conclude that although both P supply processes overlap in time, seed P was the main P source during early growth stages.





Similar content being viewed by others
References
Assuero SG, Mollier A, Pellerin S (2004) The decrease in growth of phosphorus-deficient maize leaves is related to a lower cell production. Plant Cell Environ 27:887–895
Barry DAJ, Miller MH (1989) Phosphorus nutritional-requirement of maize seedlings for maximum yield. Agron J 81:95–99
Bewley JD, Black M (1994) Seeds: physiology of development and germination. Plenum, New York
Bhadoria PS, El Dessougi H, Liebersbach H, Claassen N (2004) Phosphorus uptake kinetics, size of root system and growth of maize and groundnut in solution culture. Plant Soil 262:327–336
Bonhomme R, Derieux M, Edmeades GO (1994) Flowering of diverse maize cultivars in relation to temperature and photoperiod in multilocation field trials. Crop Sci 34:156–164
Cheng Wang I, Mitchell HL, Barham HN (1959) The phytin content of sorghum grain. Trans Kans Acad Sci 62:208–211
Colomb B, Kiniry JR, Debaeke P (2000) Effect of soil phosphorus on leaf development and senescence dynamics of field-grown maize. Agron J 92:428–435
Deleens E, Gregory N, Bourdu R (1984) Transition between seed reserve use and photosynthetic supply during development of maize seedlings. Plant Sci Lett 37:35–39
Derrick JW, Ryan MH (1998) Influence of seed phosphorus content on seedling growth in wheat: implications for organic and conventional farm management in South East Australia. Biol Agric Hortic 16:223–237
Fardeau JC (1993) Available soil phosphate—Its representation by a functional multiple compartment model. Agronomie 13:317–331
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40:305–346
Gavito ME, Miller MH (1998) Early phosphorus nutrition, mycorrhizae development, dry matter partitioning and yield of maize. Plant Soil 199:177–186
Glass ADM, Beaton JD, Bomke A (1980) Role of P in plant nutrition. In Proceedings of the Western Canada Phosphate Symposium. pp 357–368
Goss MJ, Miller MH, Bailey LD, Grant CA (1993) Root growth distribution in relation to nutrient availability and uptake. Eur J Agron 2:57–67
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Grant C, Bittman S, Montreal M, Plenchette C, Morel C (2005) Soil and fertilizer phosphorus: effects on plant P supply and mycorrhizal development. Can J Plant Sci 85:3–14
Greiner R, Egli I (2003) Determination of the activity of acidic phytate-degrading enzymes in cereal seeds. J Agric Food Chem 51:847–850
Hall JR, Hodges TK (1966) Phosphorus metabolism of germinating oat seeds. Plant Physiol 41:1459–1464
Harvey BMR, Oaks A (1974) The hydrolysis of endosperm protein in Zea mays. Plant Physiol 53:453–457
Horvatic M, Balint L (1996) Relationship among the phytic acid and protein content during maize grain maturation. J Agron Crop Sci 176:73–77
Laboure AM, Gagnon J, Lescure AM (1993) Purification and characterization of a phytase (myo-Inositol-hexakisphosphate phosphohydrolase) accumulated in maize (Zea mays) seedlings during germination. Biochem J 295:413–419
Lockhart HB, Hurt HD (1986) Nutrition of oats. St. Paul, American Association of Cereal Chemists, pp 297–308
Loewus FA, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci 150:1–19
Lorenz AJ, Scott MP, Lainkey KR (2007) Quantitative determination of phytate and inorganic phosphorus for maize breeding. Crop Sci 47:600–606
Lott JNA (1984) Accumulation of seed reserves of phosphorus and other minerals. Academic, New York, pp 139–166
Lott JNA, Greenwood JS, Batten GD (1995) Mechanisms and regulation of mineral nutrient storage during seed development. Marcel Dekker, New York, pp 215–235
Miller GA, Youngs VL, Oplinger ES (1980) Effect of available soil-phosphorus and environment on the phytic acid concentration in oats. Cereal Chem 57:192–194
Modi AT, Asanzi NM (2008) Seed performance of maize in response to phosphorus application and growth temperature is related to phytate-phosphorus occurrence. Crop Sci 48:286–297
Mollier A, Pellerin S (1999) Maize root system growth and development as influenced by phosphorus deficiency. J Exp Bot 50:487–497
Oberleas D, Harland BF (1981) Phytate content of foods—effect on dietary zinc bioavailability. J Am Diet Assoc 79:433–436
O’Dell BL, de Boland RA, Koirtyohann SR (1972) Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J Agric Food Chem 20:718–721
Ozanne PG (1980) Phosphate nutrition of plants—A general treatise. ASA, Madison, pp 559–589
Park S-H, Sung J-K, Lee S-Y, Park J-H, Lee J-Y, Jang B-C, Lee B-H, Kim T-W (2006) Early growth, carbohydrate, and phytic acid contents of germinating rice seeds under NaCl stress. Korean J Crop Sci 51:137–141
Pellerin S, Mollier A, Plenet D (2000) Phosphorus deficiency affects the rate of emergence and number of maize adventitious nodal roots. Agron J 92:690–697
Plénet D, Etchebest S, Mollier A, Pellerin S (2000a) Growth analysis of maize field crops under phosphorus deficiency—I. Leaf growth. Plant Soil 223:117–130
Plénet D, Mollier A, Pellerin S (2000b) Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant Soil 224:259–272
Pontoppidan K, Pettersson D, Sandberg AS (2007) The type of thermal feed treatment influences the inositol phosphate composition. Anim Feed Sci Technol 132:137–147
Raboy V (1997) Accumulation and storage of phosphate and minerals. Kluwer Academic, Dordrecht, pp 441–477
Raboy V, Dickinson DB (1984) Effect of phosphorus and zinc nutrition on soybean seed phytic acid and zinc. Plant Physiol 75:1094–1098
Raboy V, Below FE, Dickinson DB (1989) Alteration of maize kernel phytic acid levels by recurrent selection for protein and oil. J Hered 80:311–315
Raboy V, Noaman MM, Taylor GA, Pickett SG (1991) Grain phytic acid and protein are highly correlated in winter-wheat. Crop Sci 31:631–635
Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PPN, Sheridan WF, Ertl DS (2000) Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol 124:355–368
Raboy V, Young KA, Dorsch JA, Cook A (2001) Genetics and breeding of seed phosphorus and phytic acid. J Plant Physiol 158:489–497
Ravindran V, Ravindran G, Sivalogan S (1994) Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem 56:335–343
Reichwald K, Hatzack F (2008) Application of a modified Haug and Lantzsch method for the rapid and accurate photometrical phytate determination in soybean, wheat, and maize meals. J Agric Food Chem 56:2888–2891
Rodriguez D, Keltjens WG, Goudriaan J (1998) Plant leaf area expansion and assimilate production in wheat (Triticum aestivum L.) growing under low phosphorus conditions. Plant Soil 200:227–240
Rubio G, Sorgona A, Lynch JP (2004) Spatial mapping of phosphorus influx in bean root systems using digital autoradiography. J Exp Bot 55:2269–2280
Scaife MA, Smith R (1973) The phosphorus requirement of lettuce. II. A dynamic model of phosphorus uptake and growth. J Agric Sci 80:353–361
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Schjørring JK, Jensén P (1984) Phosphorus nutrition of barley, buckwheat and rape seedlings. I. Influence of seed-borne P levels and external P levels on growth, P content and 32P/31P-fractionation in shoots and roots. Physiol Plant 61:577–583
Simwemba CG, Hoseney RC, Varrianomarston E, Zeleznak K (1984) Certain B-vitamin and phytic acid contents of pearl-millet [Pennisetum americanum (L) Leeke]. J Agric Food Chem 32:31–34
Usuda H, Shimogawara K (1993) Phosphate deficiency in maize. III. Changes in amounts of sucrose-phosphate synthase during phosphate deprivation. Plant Physiol 102:176–176
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161:45–48
Whalley RDB, Mckell CM, Green LR (1966) Seedling vigor and the early nonphotosynthetic stage of seedling growth in grasses. Crop Sci 6:147–150
Wyss M, Brugger R, Kronenberger A, Remy R, Fimbeld R, Oesterhelt G, Lehman M, Van Loon AP (1999) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolyses): catalytic properties. Appl Environ Microbiol 65:367–373
Acknowledgement
This study was funded by a project grant from the Higher Education Commission (HEC), Pakistan and benefited from the financial support from the INRA (French National Institute for Agricultural Research). M. Nadeem thanks the Pakistan Higher Education Commission for funding his PhD studentship at the University of Bordeaux I, France. The authors acknowledge the technical help and the good advice offered by Anne Gallet-Budynek, Laurent Augusto, Alain Vives and Loïc Prud’homme. We also thank Daphne Goodfellow for revising the English. Thanks are also due to two referees for their thorough reviews and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Appendix
Appendix
Phytate extracted from endosperm and scutellum was assay by ion chromatography (IC) using a hydroxide eluent prepared by an eluent generator with a column designed to determine common inorganic anions. High performance liquid chromatography was carried out on a Dionex (Sunnyvale, CA, USA) Model ICS 2000 Ion Chromatography System with eluent generation (RFIC-EG system) equipped with an automatic gradient pump, a conductivity detector (DS6 heated conductivity cell) and an automated sampler (AS50). The eluent was automatically generated by the eluent generator module using purified water and an eluent generator cartridge (EGC II KOH P/N 058900) to produce a gradient of 12–90 mM potassium hydroxide. An eluent generator was used in conjunction with internal suppression (ASRS 300 4 mm at 179 mA). The concentration of the eluent was controlled by Chromeleon® Chromatography Data System software version 6.8. Separation was performed using an anion exchange column equipped with a guard column (Ion Pac AG11, 4 mm (P/N 044078)) to prevent sample contaminants from eluting onto the profiling analytical column (IonPac AS11, 4 mm P/N 044076). The detector was preceded by an ASRS® 2 anion self-regenerating suppressor, to suppress the background conductivity of the eluent. A standard solution containing a phytate salt (phytic acid sodium salt hydrated from rice, SIGMA product n° P8810-10 G) was prepared for the identification of solute peaks. For the AS11 column, the initial mobile phase was 12 mM potassium hydroxide. The gradient elution of KOH was generated automatically up to 90 mM. An injection volume of 20 μL was used and eluted with the KOH mobile phase at a flow rate of 0.80 ml min−1. Phytate was eluted after 23.2 min for a procedure that lasted 47 min per sample including 5 min for equilibration of the AS11 with 12 mM KOH prior to injection of the next sample. This method has a detection limit of 0.12 mg P L−1 for phytate. The instruments was calibrated with blanks and six standards prepared by dissolving phytic acid sodium salt in purified water (0, 200, 400, 800, 1000 and 2000 mg L−1 of phytic acid). The linearity of the standards was excellent (r² ≥ 0.999) with peak area evaluation over this range of concentrations. Phytate content was converted into phytate-P by dividing phytate content by 3.548 (Raboy and Dickinson 1984).
Rights and permissions
About this article
Cite this article
Nadeem, M., Mollier, A., Morel, C. et al. Relative contribution of seed phosphorus reserves and exogenous phosphorus uptake to maize (Zea mays L.) nutrition during early growth stages. Plant Soil 346, 231–244 (2011). https://doi.org/10.1007/s11104-011-0814-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0814-y