Abstract
Key message
The current review provides an updated, new insights into the regulation of transcription mediated underlying mechanisms of wheat plants to osmotic stress perturbations.
Abstract
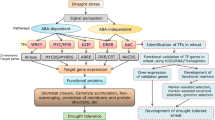
Osmotic stress tolerance mechanisms being complex are governed by multiple factors at physiological, biochemical and at the molecular level, hence approaches like “OMICS” that can underpin mechanisms behind osmotic tolerance in wheat is of paramount importance. The transcription factors (TFs) are a class of molecular proteins, which are involved in regulation, modulation and orchestrating the responses of plants to a variety of environmental stresses. Recent reports have provided novel insights on the role of TFs in osmotic stress tolerance via direct molecular links. However, our knowledge on the regulatory role TFs during osmotic stress tolerance in wheat remains limited. The present review in its first part sheds light on the importance of studying the role of osmotic stress tolerance in wheat plants and second aims to decipher molecular mechanisms of TFs belonging to several classes, including DREB, NAC, MYB, WRKY and bHLH, which have been reported to engage in osmotic stress mediated gene expression in wheat and third part covers the systems biology approaches to understand the transcriptional regulation of osmotic stress and the role of long non-coding RNAs in response to osmotic stress with special emphasis on wheat. The current concept may lead to an understanding in molecular regulation and signalling interaction of TFs under osmotic stress to clarify challenges and problems for devising potential strategies to improve complex regulatory events involved in plant tolerance to osmotic stress adaptive pathways in wheat.




Similar content being viewed by others
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi -SK (2003) Arabidopsis AtMYC2(bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Agarwal P, Agarwal PK, Joshi AJ, Reddy MK, Sopory SK (2010) Overexpression of PgDREB2A transcription factor enhances abiotic stress tolerance and activates downstream stress-responsive genes. Mol Biol Rep 37:1125–1135
Ahanger MA, Agarwal RM (2017) Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 254:1471–1486
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Akhtar M, Jaiswal A, Taj G, Jaiswal JP, Qureshi MI, Singh NK (2012) DREB1/CBF transcription factors: their structure, function and role in abiotic stress tolerance in plants. J Genet 91:385–395
Akpinar BA, Kantar M, Budak H (2015) Root precursors of microRNAs in wild emmer and modern wheats show major differences in response to drought stress. Funct Integr Genom 15(5):587–598
Akpinar BA, Biyiklioglu S, Alptekin B, Havránková M, Vrána J, Doležel J, Distelfeld A, Hernandez P, Budak IWGSC H (2018) Chromosome-based survey sequencing reveals the genome organization of wild wheat progenitor Triticum dicoccoides. Plant Biotechnol J. https://doi.org/10.1111/pbi.12940
Allen M, Yamasaki K, Ohme-TM, Tateno M, Suzuki M (1998) A novel mode of DNA recognition by a β-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. EMBO J 17:5484–5496
Alptekin B, Budak H (2017) Wheat miRNA ancestors: evident by transcriptome analysis of A, B, and D genome donors. Funct Integr Genom 17(2–3):171–187
Alptekin B, Langridge P, Budak H (2017) Abiotic stress miRNomes in the Triticeae. Funct Integr Genom 17(2–3):145–170
Anbazhagan K, Bhatnagar-MP, Vadez V, Dumbala SR, Kishor PB, Sharma KK (2015) DREB1A overexpression in transgenic chickpea alters key traits influencing plant water budget across water regimes. Plant Cell Rep 34:199–210
Ariel F, Jegu T, Latrasse D, Romero-Barrios N, Christ A, Benhamed M et al (2014) Noncoding transcription by alternative RNA polymerases dynamically regulates an auxin-driven chromatin loop. Mol Cell 55:383–396. https://doi.org/10.1016/j.molcel.2014.06.011
Atchley WR, Terhalle W, Dress A (1999) Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J Mol Evol 48:501–516
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15:2730–2741
Badawi M, Reddy YV, Agharbaoui Z, Tominaga Y, Danyluk J, Sarhan F, Houde M (2008) Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol 49:1237–1249
Baldoni E, Genga A, Cominelli E (2015) Plant MYB transcription factors: their role in drought response mechanisms. Int J Mol Sci 16:15811–15851
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Bhatia G, Goyal N, Sharma S, Upadhyayay SK, Singh K (2017) Present scenario of long non-coding RNAs in plants. Noncoding RNA 3(2), E16. https://doi.org/10.3390/ncrna3020016
Bhattacharjee A, Khurana JP, Jain M (2016) Characterization of rice homeobox genes, OsHOX22 and OsHOX24, and over-expression of OsHOX24 in transgenic arabidopsis suggest their role in abiotic stress response. Front Plant Sci 7:627
Budak H, Kantar M, Yucebilgili Kurtoglu K (2013) Drought tolerance in modern and wild wheat. Sci World J. https://doi.org/10.1155/2013/548246
Budak H, Hussain B, Khan Z, Ozturk NZ, Ullah N (2015) From genetics to functional genomics: improvement in drought signaling and tolerance in wheat. Front Plant Sci 6:1012
Cagirici HB, Alptekin B, Budak H (2017) RNA sequencing and co-expressed long non-coding RNA in modern and wild wheats. Sci Rep 7(1):10670
Cai H, Tian S, Dong H, Guo C (2015) Pleiotropic effects of TaMYB3R1 on plant development and response to osmotic stress in transgenic Arabidopsis. Gene 558:227–234
Cao ZH, Zhang SZ, Wang RK, Zhang RF, Hao YJ (2013) Genome wide analysis of the apple MYB transcription factor family allows the identification of Mdo MYB121 gene confering abiotic stress tolerance in plants. PLoS ONE 8:e69955
Cech TR, Stetiz (2014) The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157:77–94
Chandra K, Prasad R, Thakur P, Madhukar K, Prasad LC (2017) Heat tolerance in wheat-A key strategy to combat climate change through molecular markers. Int J Curr Microbiol Appl Sci 6:662–675
Chang H, Chen D, Kam J, Richardson T, Drenth J, Guo X, McIntyre CL, Chai S, Rae AL, Xue GP (2016) Abiotic stress upregulated TaZFP34 represses the expression of type-B response regulator and SHY2 genes and enhances root to shoot ratio in wheat. Plant Sci 252:88–102
Charfeddine M, Saïdi MN, Charfeddine S, Hammami A, Gargouri BR (2014) Genome-wide analysis and expression profiling of the ERF transcription factor family in potato (Solanum tuberosum L.). Mol Biotechnol 57:348–358
Chen D, Yuan C, Zhang J, Zhang Z, Bai L, Meng Y, Chen LL, Chen M (2012) PlantNATsDB: a comprehensive database of plant natural antisense transcripts. Nucleic Acids Res 40:D1187–D1193
Chen M, Zhao Y, Zhuo C, Lu S, Guo Z (2015) Over-expression of a NF-YC transcription factor from bermuda grass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol J 13:482–491
Chen L, Han J, Deng X, Tan S, Li L, Li L, Zhou J, Peng H, Yang G, He G, Zhang W (2016) Expansion and stress responses of AP2/EREBP superfamily in Brachypodium distachyon. Sci Rep 6:21623
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Christianson JA, Dennis ES, Llewellyn DJ, Wilson IW (2010) ATAF NAC transcription factors: regulators of plant stress signaling. Plant Signal Behav 5:428–432
Chuck G, Meeley RB, Hake S (1998) The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev 12:1145–1154
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163. https://doi.org/10.1186/1471-2229-11-163
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143:1739–1751
Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J et al (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17:268–281
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Denekamp M, Smeekens SC (2003) Integration of wounding and osmotic stress signals determines the expression of the AtMYB102 transcription factor gene. Plant Physiol 132:1415–1423
Deng KJ, Zhou JP, Wu XH, Sun G, Wang T, Tang AT et al (2016) Polyamine plays key role in different osmotic stress responses of wheat-rye 1bl/1rs translocation lines. Cereal Res Commun 44:549–560
Deng F, Zhang X, Wang W, Yuan R, Shen F (2018) Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol 18:23. https://doi.org/10.1186/s12870-018-1238-0
Ding J, Lu Q, Ouyang Y, Mao H, Zhang P, Yao J, Xu C, Li X, Xiao J, Zhang Q (2012) A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci USA 109:2654–2659
Dinh TT, Girke T, Liu X, Yant L, Schmid M, Chen X (2012) The floral homeotic protein APETALA2 recognizes and acts through an AT-rich sequence element. Development 139:1978–1986
Du H, Yang S-S, Liang Z, Feng BR, Liu L, Huang YB et al (2012) Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol 1:1–22
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581
Ergen NZ, Budak H (2009) Sequencing over 13 000 expressed sequence tags from six subtractive cDNA libraries of wild and modern wheats following slow drought stress. Plant Cell Environ 32(3):220–236
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
FAO (2017) FAO cereal supply and demand brief. http://www.fao.org/worldfoodsituation/csdb/en/. Accessed 24 Sept 2017
Feller A, Machemer K, Braun EL, Grotewold E (2011) Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J 66:94–116
Feng K, Nie X, Cui L, Deng P, Wang M, Song W (2017) Genome-wide identification and characterization of salinity stress-responsive miRNAs in wild emmer wheat (Triticum turgidum ssp. dicoccoides). Genes 8:156
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Gahlaut V, Jaiswal V, Kumar A, Gupta PK (2016) Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.). Theor Appl Genet 129:2019–2042
Gallart AP, Pulido AH, de Lagrán IA, Sanseverino W, Cigliano RA (2016) GREENC: a Wiki-based database of plant lncRNAs. Nucleic Acids Res 44:D1161–D1166
Gao J, Zhang Z, Peng R, Xiong A, Xu J et al (2010) Forced expression of MdMYB10, a myb transcription factor from apple, enhances tolerance to osmotic stress in transgenic Arabidopsis. Mol Biol Rep 38:205–211
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Genc Y, Oldach K, Verbyla AP, Lott G, Hassan M, Tester M, Wallwork H, McDonald GK (2010) Sodium exclusion QTL associated with improved seedling growth in bread wheat under salinity stress. Theor Appl Genet 121(5):877–894
Glover-Cutter KM, Alderman S, Dombrowski JE, Martin RC (2014) Enhanced oxidative stress resistance through activation of a zinc deficiency transcription factor in Brachypodium distachyon. Plant Physiol 166:1492–1505
Goyal E, Amit SK, Singh RS, Mahato AK, Chand S et al (2016) Transcriptome profiling of the salt-stress response in Triticum aestivum cv. Kharchia local. Sci Rep 6:27752
Gu C, Guo ZH, Hao PP, Wang G-M, Jin ZM et al (2017) Multiple regulatory roles of AP2/ERF transcription factors in angiosperm. Bot Stud 58:6
Guo XJ, Wang JR (2017) Global identification, structural analysis and expression characterization of bHLH transcription factors in wheat. BMC Plant Biol 17:90
Guo M, Liu JH, Ma X, Luo DX, Gong ZH et al (2016) The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front Plant Sci 7:114
Gupta OP, Meena NL, Sharma I, Sharma P (2014) Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol Biol Rep 41(7):4623–4629
Gupta BK, Tripathi AK, Joshi R, Pareek A, Singla-Pareek SL (2015) Designing climate-smart future crops employing signal transduction components. In: Pandey GK (ed) Elucidation of abiotic stress signaling in plants: Functional genomics perspectives, vol 2. Springer, New York, pp 393–414
Gupta B, Joshi R, Pareek A, Singla-Pareek SL (2017) Transgenic approaches to improve crop productivity via phytohormonal research: a focus on the mechanisms of phytohormone action. In: Pandey GK (ed) Mechanism of plant hormone signaling under stress: a functional genomic frontier, 2nd vol. Wiley, Hoboken, pp 533–567
Hasanuzzaman M, Nahar K, Rahman A, Anee TI, Alam MU, Bhuiyan TF, Oku H, Fujita M (2017) Approaches to enhance salt stress tolerance in wheat. In: Wanyera R (ed) Wheat improvement, management and utilization. InTech, Rijeka
He Q, Jones DC, Li W, Xie F, Ma J et al (2016) Genome-wide identification of R2R3-MYB genes and expression analyses during abiotic stress in Gossypium raimondii. Sci Rep 6:22980
Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B et al (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20:735–747
Hernandez JA, Ferrer MA, Jimenez A, Barcelo AR, Sevilla F (2001) Antioxidant systems and O2 –/H2O2 production in the apoplast of pea leaves, its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Hong JC (2016) General aspects of plant transcription factor families A2. In: Gonzalez DH (ed) Plant transcription factors. Academic Press, Boston, pp 35–56
Hong Y, Jackson S (2015) Floral induction and flower formation- the role and potential applications of miRNAs. Plant Biotechnol J 13:282–292. https://doi.org/10.1111/pbi.12340
Hong Y, Pan X, Welti R, Wang X (2008) Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell 20:803–816
Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14:660–668
Huang D, Feurtado JA, Smith MA, Flatman LK, Koh C, Cutler AJ (2017) Long noncoding miRNA gene represses wheat β-diketone waxes. Proc Nat Acad Sci. https://doi.org/10.1073/pnas.1617483114
Hussain B, Lucas SJ, Ozturk L, Budak H (2017) Mapping QTLs conferring salt tolerance and micronutrient concentrations at seedling stage in wheat. Sci Rep 7(1):15662
Jabnoune M, Secco D, Lecampion C, Robaglia C, Shu Y, Poirier Y (2013) A rice cis-natural antisense RNA acts as a translational enhancer for its cognate mRNA and contributes to phosphate homeostasis and plant fitness. Plant Cell 25:4166–4182. https://doi.org/10.1105/tpc.113.116251
James RA, Davenport RJ, Munns R (2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol 142(4):1537–1547
Jensen MK, Kjaersgaard T, Nielsen MM, Galberg P, Petersen K et al (2010) The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signaling. Biochem J 426:183–196
Jiang J, Ma S, Ye N, Jiang M, Cao J et al (2017) WRKY transcription factors in plant responses to stresses. J Int Plant Biol 59:86–101
Jin J, Liu J, Wang H, Wong L, Chua NH (2013) PLncDB: plant long non-coding RNA database. Bioinformatics 29:1068–1071
Jofuku KD, Omidyar PK, Gee Z, Okamuro JK (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA 102:3117–3122
Joshi R, Karan R (2014) Physiological, biochemical and molecular mechanisms of drought tolerance in plants. In: Gaur RK, Sharma P (eds) Molecular approaches in plant abiotic stress. CRC Press, Boca Raton, pp 209–231
Joshi R, Singh B, Bohra A, Chinnusamy V (2015) Salt stress signalling pathways: specificity and crosstalk. In: Wani SH, Hossain MA (eds) Managing salinity tolerance in plants: molecular and genomic perspectives. CRC Press, Boca Raton, pp 51–78.
Joshi R, Wani SH, Singh B, Bohra A, Dar ZA, Lone AA et al (2016) Transcription factors and plants response to drought stress: current understanding and future directions. Front Plant Sci 7:1029
Joshi R, Anwar K, Das P, Singla-Pareek SL, Pareek A (2017) Overview of methods for assessing salinity and drought tolerance of transgenic wheat lines. In: Bhalla P, Singh M (eds) Wheat biotechnology. Methods in molecular biology, vol 1679. Humana Press, New York, pp 83–95
Joshi R, Sahoo KK, Tripathi AK, Kumar R, Gupta BK et al (2018) Knockdown of an inflorescence meristem-specific cytokinin oxidase—OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ 41(5):936–946
Kajla M, Yadav VK, Khokhar J, Singh S, Chhokar RS, Meena RP et al (2015) Increase in wheat production through management of abiotic stresses: a review. J Appl Nat Sci 7:1070–1080
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kaur V, Singh S, Behl R (2016) Heat and drought tolerance in wheat: integration of physiological and genetic platforms for better performance under stress. Ekin J Crop Breed Genet 2:1–14
Kaur A, Gupta OP, Meena NL, Grewal A, Sharma P (2017) Comparative temporal expression analysis of microRNAs and their target genes in contrasting wheat genotypes during osmotic stress. Appl Biochem Biotechnol 181:613–626
Kawa D, Testerink C (2017) Regulation of mRNA decay in plant responses to salt and osmotic stress. Cell Mol Life Sci 74:1165–1176
Keunen E, Peshev D, Vangronsveld J, Van Den Ende W, Cuypers A (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255
Khan MN, Mobin M, Abbas ZK, Siddiqui MH (2017) Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 68:91–102
Khare T, Kumar V, Kavi Kishor PB (2015) Na+ and Cl– ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 252:1149–1165
Kim YS, Kim SG, Park JE, Park HY, Lim MH, Chua NH et al (2006) A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18:3132–3144
Kim SG, Lee S, Seo PJ, Kim SK, Kim JK et al (2010) Genome-scale screening and molecular characterization of membrane-bound transcription factors in Arabidopsis and rice. Genomics 95:56–65
Kim D, Alptekin B, Budak H (2018) CRISPR/Cas9 genome editing in wheat. Funct Integr Genom 18(1):31–41
Kinoshita T, Seki M (2014) Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol 55:1859–1863. https://doi.org/10.1093/pcp/pcu125
Komatsu S, Kamal AHM, Hossain Z (2014) Wheat proteomics: proteome modulation and abiotic stress acclimation. Front Plant Sci 5:684
Kornienko AE, Guenzl PM, Barlow DP, Pauler FM (2013) Gene regulation by the act of long non-coding RNA transcription. BMC Biol 11:59
Kristensen PS, Jahoor A, Andersen JR, Cericola F, Orabi J, Janss L, Jensen J (2018) Genome-wide association studies and comparison of models and cross-validation strategies for genomic prediction of quality traits in advanced winter wheat breeding lines. Front Plant Sci 9:69
Kumar A, Kumar S, Kumar U, Suravajhala P, Gajula MNVP (2016) Functional and structural insights into novel DREB1A transcription factors in common wheat (Triticum aestivum L.): a molecular modeling approach. Comput Biol Chem 64:217–226
Kumar V, Khare T, Sharma M, Wani SH (2017) ROS induced signaling and gene-expression in crops under salinity stress. In: Khan MIR (ed) Reactive oxygen species and antioxidant systems: role and regulation under abiotic stress. Springer, Singapore, pp 159–184
Kumar V, Khare T, Shriram V, Wani SH (2018) Plant small RNAs: the essential epigenetic regulators of gene expression for salt-stress responses and tolerance. Plant Cell Rep 37:61–75. https://doi.org/10.1007/s00299-017-2210-4
Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M et al (2012) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40:D1202–D1210
Lasram A, Masmoudi MM, Mechlia NB (2017) Effect of high temperature stress on wheat and barley production in Northern Tunisia. In: Ouessar M, Gabriels D, Tsunekawa A, Evett S. (eds) Water and land security in drylands. Springer, Cham
Lee SJ, Kang JY, Park HJ, Kim MD, Bae MS, Choi HI et al (2010) DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol 153:716–727
Lee SH, Woo SY, Je SM (2015) Effects of elevated CO2 and water stress on physiological responses of Perilla frutescens var. japonica HARA. Plant Growth Regul 75:427–434
Li Q, Zhang C, Li J, Wang L, Ren Z (2012) Genome-wide identification and characterization of R2R3 MYB family in Cucumis sativus. PLoS ONE 7:e47576
Li Z, Peng R, Tian Y, Han H, Xu J, Yao Q (2016) Genome-wide identification and analysis of the MYB transcription factor superfamily in Solanum lycopersicum. Plant Cell Physiol 57:1657–1677
Lippold F, Sanchez DH, Musialak M, Schlereth A, Scheible WR, Hincha DK et al (2009) AtMyb41 regulates transcriptional and metabolic responses to osmotic stress in Arabidopsis. Plant Physiol 149:1761–1772
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K et al (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA-binding domain separate two cellular signal transduction pathways in drought and low temperature-responsive gene expression in Arabidopsis. Plant Cell 10:1391–1406
Liu H, Tian X, Li Y, Wu CA, Zheng C (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14:836–843
Liu Z, Xin M, Qin J, Peng H, Ni Z, Yao Y et al (2015a) Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.). BMC Plant Biol 15:152
Liu J, Wang H, Chua NH (2015b) Long noncoding transcriptome of plants. Plant Biotechnol J 13:319–328
Ma K, Shi W, Xu M, Liu J, Zhang F (2018) Genome-wide identification and characterization of long non-coding RNA in wheat roots in response to Ca2+ channel blocker. Front Plant Sci 9:244. https://doi.org/10.3389/fpls.2018.00244
Mao X, Chen S, Li A, Zhai C, Jing R (2014) Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis. PLoS ONE 10:e84359
Marcin´ska I, Czyczyło-Mysza I, Skrzypek E, Filek M, Grzesiak S, Grzesiak MT et al (2013) Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol Plant 35:451–461
Mattick JS, Rinn JL (2015) Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol 22:5–7
McIntyre CL, Rattey A, Kilian A, Dreccer MF, Shorter R (2014) Preferential retention of chromosome regions in derived synthetics wheat lines: a source of novel alleles for wheat improvement. Crop Pasture Sci 65:125–138
Miao Y, Laun TM, Smykowski A, Zentgraf U (2007) Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol 65:63–76
Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Gen 16:237–251
Mickky BM, Aldesuquy HS (2017) Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt J Bas Appl Sci 4:47–54
Mittal V, Singh N, Narwal S, Mamrutha H, Tiwari V, Sharma I (2015) Effect of osmotic stress on root architecture and defensive system in wheat genotypes at seedling stage. J Wheat Res 7(2):52–59
Moller IM, Sweetlove LJ (2010) ROS signalling-specificity is required. Trends Plant Sci 15:370–374
Moose SP, Sisco PH (1996) Glossy15, an APETALA2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev 10:3018–3027
Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, Ismagul A et al (2011) Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol J 9:230–249
Muthusamy SK, Dalal M, Chinnusamy V, Bansal KC (2016) Differential regulation of genes coding for organelle and cytosolic ClpATPases under biotic and abiotic stresses in wheat. Front Plant Sci 7:929
Naderi R, Valizadeh M, Toorchi M, Shakiba MR (2014) Antioxidant enzyme changes in response to osmotic stress in wheat (Triticum aestivum L.) seedling. Acta Biol Szeged 58:95–101
Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819:97–103
Oda-Yamamizo C, Mitsuda N, Sakamoto S, Ogawa D, Ohme-Takagi M, Ohmiya A (2016) The NAC transcription factor ANAC046 is a positive regulator of chlorophyll degradation and senescence in Arabidopsis leaves. Sci Rep 6:23609
Ohto MA, Floyd SK, Fischer RL, Goldberg RB, Harada JJ (2009) Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex Plant Reprod 22:277–289
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K et al (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10:239–247
Pandey B, Sharma P, Saini M, Pandey DM, Sharma M (2014) Isolation and characterization of dehydration-responsive element-binding factor 2 (DREB2) from Indian wheat (T. aestivum L.) cultivars. Aust J Crop Sci 8:44–45
Pandey R, Bhardwaj AR, Agarwal M, Katiyar-Agarwal S (2017) Discovery of small RNAs in wheat: a survey. Ind J Plant Physiol 1:1–1
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6:69
Pieri A, Pè ME, Bertolini E (2018) Long non-coding RNAs in wild wheat progenitors. bioRxiv 1:301804
Pires N, Dolan L (2010) Origin and diversification of basic-helix-loop-helix proteins in plants. Mol Biol Evol 27:862–874
PLncRNAdb Ming Chen’s Lab. http://bis.zju.edu.cn/PlncRNADB/index.php. Accessed 3 Jun 2018
Poersch-Bortolon LB, Pereira JF, Nhani JA, Gonzáles HHS, Torres GAM, Consoli L (2016) Gene expression analysis reveals important pathways for drought response in leaves and roots of a wheat cultivar adapted to rainfed cropping in the Cerrado biome. Gen Mol Biol 39:629–645
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136:629–641
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Qayyum A, Razzaq A, Bibi Y, Khan SU, Abbasi KS, Sher A, Mehmood A, Ahmed W, Mahmood I, Manaf A, Khan A (2018) Water stress effects on biochemical traits and antioxidant activities of wheat (Triticum aestivum L.) under In vitro conditions. Acta Agric Scand B 68(4):283–290
Qin Y, Tian Y, Liu X (2015) A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem Biophy Res Comm 464:428–433
Qiu Z, Yuan M, He Y, Li Y, Zhang L (2017) Physiological and transcriptome analysis of He-Ne laser pretreated wheat seedlings in response to drought stress. Sci Rep 7:6108
Quek XC, Thomson DW, Maag JL, Bartonicek N, Signal B, Clark MB, Gloss BS, Dinger ME (2014) lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res 43:gku988
Rabara RC, Tripathi P, Rushton PJ (2014) The potential of transcription factor-based genetic engineering in improving crop tolerance to drought. OMICS: J Integr Biol 18(10):601–614
Rahaie M, Gomarian M, Alizadeh H, Malboobi MA, Naghavi MR (2011) The expression analysis of transcription factors under long term salt stress in tolerant and susceptible wheat genotypes using reverse northern blot technique. Iran J Crop Sci 13:580–595
Reynolds M, Bonnett D, Chapman SC, Furbank RT, Manès Y, Mather DE et al (2010) Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J Exp Bot 62:439–452
Rinerson CI, Rabara RC, Tripathi P, Shen QJ, Rushton PJ (2015) The evolution of WRKY transcription factors. BMC Plant Biol 15:66
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Rizvi A, Khan MS (2017) Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere 185:942–955
RNAcentral Consortium (2016) RNAcentral: a comprehensive database of non-coding RNA sequences. Nucleic Acids Res 45:gkw1008
Rong W, Qi L, Wang A, Ye X, Du L, Liang H et al (2014) The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12:468–479
Sablowski RW, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92:93–103
Saleh A, Lumreras V, Pages M (2005) Functional role of DRE binding transcription factors in abiotic stress. In: Tuberosa R, Phillips RL, Gale M (eds) Proceedings of the international congress ‘In the Wake of the Double Helix From the Green Revolution to the Gene Revolution’, 27–31 May 2003, Bologna, Italy, pp 193–205, Avenue media. Italy, Bologna
Shang H, Li W, Zou C, Yuan Y (2013) Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr.: chromosomal location, structure, phylogeny, and expression patterns. J Integr Plant Biol 55(7):663–676
Shao H, Wang H, Tang X (2015) NAC transcription factors in plant multiple abiotic stress responses: progress and prospects. Front Plant Sci 6:902
Shao Y, Wei J, Wu F, Zhang H, Yang D, Liang Z, Jin W (2016) DsTRD: Danshen Transcriptional Resource Database. PLoS ONE 11:e0149747
Sharma MK, Kumar R, Solanke AU, Sharma R, Tyagi AK, Sharma AK (2010) Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol Genet Genom 284:455–475
Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T et al (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52:344–360
Shen YG, Zhang WK, He SJ, Zhang JS, Liu Q, Chen SY (2003) An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor Appl Genet 106:923–930
Shoeva OY, Gordeeva EI, Arbuzova VS, Khlestkina EK (2017) Anthocyanins participate in protection of wheat seedlings from osmotic stress. Cereal Res Commun 45:47–56
Shriram V, Kumar V, Devarumath RM, Khare T, Wani SH (2016) MicroRNAs as potent targets for abiotic stress tolerance in plants. Front Plant Sci 7:817. https://doi.org/10.3389/fpls.2016.00817
Shu Y, Liu Y, Zhang J, Song L, Guo C (2015) Genome-wide analysis of the AP2/ERF superfamily genes and their responses to abiotic stress in Medicago truncatula. Front Plant Sci 6:1247
Shumayla, Sharma S, Taneja M, Tyagi S, Singh K, Upadhyay SK (2017) Survey of high throughput RNA-Seq data reveals potential roles for lncRNAs during development and stress response in bread wheat. Front Plant Sci 8:1019. https://doi.org/10.3389/fpls.2017.01019
Simionato E, Ledent V, Richards G, Thomas-CM, Kerner P, Coornaert D et al (2007) Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evol Biol 7:33
Singh B, Bohra A, Mishra S, Joshi R, Pandey S (2015) Embracing new-generation ‘omics’ tools to improve drought tolerance in cereal and food-legume crops. Biol Plant 59:413–428
Singh B, Mishra S, Bohra A, Joshi R, Siddique KHM (2018) Crop phenomics for abiotic stress tolerance in crop plants. In: Wani SH (ed) Biochemical, physiological and molecular avenues for combating abiotic stress tolerance in plants. Elsevier, New York
Song X, Sun L, Luo H, Ma Q, Zhao Y, Pei D (2016) Genome-wide identification and characterization of long non-coding RNAs from mulberry (Morus notabilis) RNA-seq Data. Genes 7:11
Souer E, Van HA, Kloos D, Mol J, Koes R (1996) The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85:159–170
Srivastava R, Deng Y, Howell SH (2014) Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front Plant Sci 5:59
Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4:447–456
Sun G (2012) MicroRNAs and their diverse functions in plants. Plant Mol Biol 80:17–36
Sunkar R, Li YF, Jagadeeswaran G (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17:196–203. https://doi.org/10.1016/j.tplants.2012.01.010
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sweetlove LJ, Foyer CH (2004) Roles for reactive oxygen species and antioxidants in plant mitochondria. In: Day DA, Millar AH, Whelan J (eds) Plant mitochondria: from genome to function, vol 1. Advances in photosynthesis and respiration. Kluwer Academic Press, Dordrecht, pp 307–320
Szcze´sniak MW, Rosikiewicz W, Makałowska I (2016) CANTATAdb: a collection of plant long non-coding RNAs. Plant Cell Physiol 57:e8
Tabassum T, Farooq M, Ahmad R, Zohaib A, Wahid A (2017) Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol Biochem 118:362–369
Takada S, Hibara K, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128:1127–1135
Takahashi F, Tilbrook J, Trittermann C, Berger B, Roy SJ, Seki M et al (2015) Comparison of leaf sheath transcriptome profiles with physiological traits of bread wheat cultivars under salinity stress. PLoS ONE 10:e0133322
Tian J, Song Y, Du Q, Yang X, Ci D, Chen J, Xie J, Li B, Zhang D (2016) Population genomic analysis of gibberellin-responsive long non-coding RNAs in Populus. J Exp Bot 67:erw057
Tran LS, Nishiyama R, Yamaguchi-SK, Shinozaki K (2010) Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach. GM Crops 1:32–39
Tripathi P, Rabara RC, Rushton PJ (2013) A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 239:1–12
Tripathi A, Goswami K, Sanan-Mishra N (2015) Role of bioinformatics in establishing microRNAs as modulators of abiotic stress responses: the new revolution. Front Physiol 6:286. https://doi.org/10.3389/fphys.2015.00286
Trujillo LE, Sotolongo M, Menendez C, Ochogavia ME, Coll Y, Hernandez I et al (2008) SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol 49:512–525
Upadhyay J, Joshi R, Singh B, Bohra A, Vijayan R, Bhatt M, Bisht SPS, Wani SH (2017) Application of bioinformatics in understanding of plant stress tolerance. In: Hakeem K, Malik A, Vardar-Sukan F, Ozturk M (eds) Plant bioinformatics: decoding the phyta. Springer, Cham, pp 347–374
Vannini C, Locatelli F, Bracale M, Magnani E, Marsoni M et al (2004) Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J 37:115–127
Wang HLV, Chekanova JA (2017) Long noncoding RNAs in plants. In: Rao M (ed) Long non coding RNA biology. Advances in experimental medicine and biology, vol 1008. Springer, Singapore
Wang C, Deng P, Chen L, Wang X, Ma H, Hu W et al (2013) A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS ONE 8:65120
Wang X, Zeng J, Li Y, Rong X, Sun J, Sun T et al (2015a) Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front Plant Sci 6:179
Wang H, Niu QW, Wu H-W, Liu J, Ye J, Yu N, Chua N-H (2015b) Analysis of non-coding transcriptome in rice and maize uncovers roles of conserved lncRNAs associated with agriculture traits. Plant J 84:404–416
Wang TZ, Liu M, Zhao MG, Chen R, Zhang WH (2015c) Identification and characterization of long non-coding RNAs involved in osmotic and salt stress in Medicago truncatula using genome-wide high-throughput sequencing. BMC Plant Biol 15:131
Wang W, Yuan Y, Yang C, Geng S, Quan S, Long L, Cai C et al (2016). Characterization, expression, and functional analysis of a novel NAC gene associated with resistance to verticillium wilt and abiotic stress in cotton. G3 (Bethesda) 6:3951–3961
Wani SH, Sah SK, Hossain MA, Kumar V, Balachandran SM (2016) Transgenic approaches for abiotic stress tolerance in crop plants. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Advances in plant breeding strategies: agronomic, abiotic and biotic stress traits. Springer International Publishing, Cham, pp. 345–396
Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM (2009) Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol 149:981–993
Wilusz JE, Sunwoo H, Spector DL (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23:1494–1504
Wu LJ, Chen XL, Ren HY, Zhang ZJ, Zhang HW, Wang JY et al (2007) ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 226:815–825
Wu J, Zhang Z, Zhang Q, Liu Y, Zhu B, Cao J et al (2015a) Generation of wheat transcription factor FOX rice lines and systematic screening for salt and osmotic stress tolerance. PLoS ONE 10(7):e0132314
Wu H, Lv H, Li L, Liu J, Mu S, Li X et al (2015b) Genome-wide analysis of the AP2/ERF transcription factors family and the expression patterns of DREB genes in Moso Bamboo (Phyllostachys edulis). PLoS ONE 10:e0126657
Wunderlich M, Groß-Hardt R, Schöffl F (2014) Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol Biol 85:541–550. https://doi.org/10.1007/s11103-014-0202-0
Xie C, Yuan J, Li H, Li M, Zhao G, Bu D, Zhu W, Wu W, Chen R, Zhao Y (2014) NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res 42:D98–D103
Xin M, Wang Y, Yao Y, Song N, Hu Z, Qin D, Xie C, Peng H, Ni Z, Sun Q (2011) Identification and characterization of wheat long non-protein coding RNAs responsive to powdery mildew infection and heat stress by using microarray analysis and SBS sequencing. BMC Plant Biol 11(1):61
Xuan H, Zhang L, Liu X, Han G, Li J, Li X, Liu A, Liao M, Zhang S (2015) PLNlncRbase: a resource for experimentally identified lncRNAs in plants. Gene 573:328–332
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot 63:2541–2556
Yang T, Yao S, Hao L, Zhao Y, Lu W, Xiao K (2016) Wheat bHLH-type transcription factor gene TabHLH1 is crucial in mediating osmotic stresses tolerance through modulating largely the ABA-associated pathway. Plant Cell Rep 35:2309–2323
Yanhui C, Xiaoyuan Y, Kun H, Meihua L, Jigang L, Zhaofeng G et al (2006) The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol 60:107–124
Yi X, Zhang Z, Ling Y, Xu W, Su Z (2015) PNRD: a plant non-coding RNA database. Nucleic Acids Res 43:D982–D989
Zhai Y, Zhang L, Xia C, Fu S, Zhao G, Jia J et al (2016) The wheat transcription factor, TabHLH39, improves tolerance to multiple abiotic stressors in transgenic plants. Biochem Biophys Res Comm 473:1321–1327
Zhang Z, Huang R (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 73:241–249
Zhang G, Chen M, Chen X, Xu Z, Guan S, Li LC et al (2008) Phylogeny, gene structures, and expression patterns of the ERF gene family in soybean (Glycine max L.). J Exp Bot 59:4095–4107
Zhang N, Yang J, Wang Z, Wen Y, Wang J, He W, Liu B, Si H, Wang D (2014) Identification of novel and conserved microRNAs related to drought stress in potato by deep sequencing. PLoS ONE 9:e95489
Zhang L, Zhang L, Xia C, Zhao G, Liu J, Jia J et al (2015) A novel wheat bZIP transcription factor, TabZIP60, confers multiple abiotic stress tolerances in transgenic Arabidopsis. Physiol Plant 153:538–554
Zhang LN, Zhang L, Xia C, Zhao G, Jia J, Kong X (2016) The novel wheat transcription factor TaNAC47 enhances multiple abiotic stress tolerances in transgenic plants. Front Plant Sci 6:1174
Zhang C, Tang G, Peng X, Peng X, Sun F, Liu S, Xi Y (2018) Long non-coding RNAs of switchgrass (Panicum virgatum L.) in multiple dehydration stressses. BMC Plant Biol 18:79. https://doi.org/10.1186/s12870-018-1288-3
Zhong R, Lee C, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3:1087–1103
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61:4157–4168
Zhou B, Zhao H, Yu J, Guo C, Dou X, Song F, Hu G, Cao Z, Qu Y, Yang Y, Zhou Y, Wang J (2018) EVLncRNAs: a manually curated database for long non-coding RNAs validated by low-throughput experiments. Nucleic Acids Res 46:D100–D105. https://doi.org/10.1093/nar/gkx677
Zhu J, Hao P, Chen G, Han C, Li X, Zeller FJ et al (2014) Molecular cloning, phylogenetic analysis, and expression profiling of endoplasmic reticulum molecular chaperone BiP genes from bread wheat (Triticum aestivum L.). BMC Plant Biol 14:260
Zhuang J, Cai B, Peng RH, Zhu B, Jin XF, Xue Y et al (2008) Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa. Biochem Biophys Res Commun 371:468–474
Zou C, Wang Q, Lu C, Yang W, Zhang Y, Cheng H, Feng X, Prosper MA, Song G (2016) Transcriptome analysis reveals long noncoding RNAs involved in fiber development in cotton (Gossypium arboreum). Sci China Life Sci 59:164–171
Acknowledgements
Author SHW is thankful to (UGC) New Delhi, India for the award of Raman post-doctoral fellowship, Abbu Zaid is thankful to Aligarh Muslim University, Aligarh and UGC-New Delhi India for financial assistance in the form of research fellowship No. BTM-2015-04-GH-7403. RJ acknowledges Dr. D S Kothari Postdoctoral Fellowship from UGC, Government of India. The research in VK’s lab is supported through the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India funds (Grant Nos. SR/FT/LS-93/2011, EMR/2016/003,896).
Author information
Authors and Affiliations
Contributions
SHW conceived the idea. SHW, RJ, PT, AZ, AK, VK, GC, JU, MB wrote the manuscript. RJ and SHW critically analyzed and revised the whole manuscript. All authors read and approved the final manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Wani, S.H., Tripathi, P., Zaid, A. et al. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol Biol 97, 469–487 (2018). https://doi.org/10.1007/s11103-018-0761-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0761-6