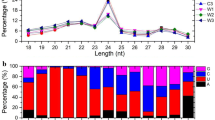
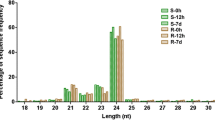
Abstract
Populus euphratica, a typical hydro-halophyte, is ideal for studying salt stress responses in woody plants. MicroRNAs (miRNAs) are endogenous non-coding small RNAs that fulfilled an important post-transcriptional regulatory function. MiRNA may regulate tolerance to salt stress but this has not been widely studied in P. euphratica. In this investigation, the small RNAome, degradome and transcriptome were studied in salt stress treated P. euphratica by deep sequencing. Two hundred and eleven conserved miRNAs between Populus trichocarpa and P. euphratica have been found. In addition, 162 new miRNAs, belonging to 93 families, were identified in P. euphratica. Degradome sequencing experimentally verified 112 targets that belonged to 51 identified miRNAs, few of which were known previously in P. euphratica. Transcriptome profiling showed that expression of 15 miRNA-target pairs displayed reverse changing pattern under salt stress. Together, these results indicate that, in P. euphratica under salt stress, a large number of new miRNAs could be discovered, and both known and new miRNA were functionally cleaving to their target mRNA. Expression of miRNA and target were correspondingly induced by salt stress but that it was a complex process in P. euphratica.





Similar content being viewed by others
References
Addo-Quaye C, Eshoo TW, Bartel DP, Axtell MJ (2008) Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr Biol 18:758–762
Addo-Quaye C, Miller W, Axtell MJ (2009) CleaveLand: a pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 25:130–131
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121:207–221
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7:986–995
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Brinker M, Brosche M, Vinocur B, Abo-Ogiala A, Fayyaz P et al (2010) Linking the salt transcriptome with physiological responses of a salt-resistant Populus species as a strategy to identify genes important for stress acclimation. Plant Physiol 154:1697–1709
Brosche M, Vinocur B, Alatalo ER, Lamminmaki A, Teichmann T et al (2005) Gene expression and metabolite profiling of Populus euphratica growing in the Negev desert. Genome Biol 6:R101
Chang Y, Chen S, Yin W, Wang RG, Liu YF et al (2006) Growth, gas exchange, abscisic acid, and calmodulin response to salt stress in three poplars. J Integr Plant Biol 48(3):286–293
Chen S, Polle A (2010) Salinity tolerance of Populus. Plant Biol (Stuttg) 12:317–333
Chen SL, Li JK, Wang SS, Huttermann A, Altman A (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees Struct Funct 15:186–194
Chen J, Xia X, Yin W (2009) Expression profiling and functional characterization of a DREB2-type gene from Populus euphratica. Biochem Biophys Res Commun 378:483–487
Cuperus JT, Fahlgren N, Carrington JC (2011) Evolution and functional diversification of MIRNA genes. Plant Cell 23:431–442
Ding M, Hou P, Shen X, Wang M, Deng S et al (2010) Salt-induced expression of genes related to Na(+)/K(+) and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol Biol 73:251–269
Engstrom EM, Andersen CM, Gumulak-Smith J, Hu J, Orlova E et al (2011) Arabidopsis homologs of the petunia hairy meristem gene are required for maintenance of shoot and root indeterminacy. Plant Physiol 155:735–750
Fukuda A, Nakamura A, Tanaka Y (1999) Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta 1446:149–155
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ et al (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98:11444–11449
German MA, Luo S, Schroth G, Meyers BC, Green PJ (2009) Construction of Parallel Analysis of RNA Ends (PARE) libraries for the study of cleaved miRNA targets and the RNA degradome. Nat Protoc 4:356–362
Gu R, Fonseca S, Puskas LG, Hackler L Jr, Zvara A et al (2004) Transcript identification and profiling during salt stress and recovery of Populus euphratica. Tree Physiol 24:265–276
Guan LM, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22:87–95
Hudson ME, Lisch DR, Quail PH (2003) The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J 34:453–471
Janz D, Behnke K, Schnitzler JP, Kanawati B, Schmitt-Kopplin P, et al (2010) Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol 10:150
Junghans U, Polle A, Duchting P, Weiler E, Kuhlman B et al (2006) Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant Cell Environ 29:1519–1531
Kawashima CG, Yoshimoto N, Maruyama-Nakashita A et al (2009) Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 57(2):313–321
Lenz D, May P, Walther D (2011) Comparative analysis of miRNAs and their targets across four plant species. BMC Res Notes 4:483
Li B, Yin W, Xia X (2009) Identification of microRNAs and their targets from Populus euphratica. Biochem Biophys Res Commun 388(2):272–277
Li B, Qin Y, Duan H, Yin W, Xia X (2011a) Genome-wide characterization of new and drought stress responsive microRNAs in Populus euphratica. J Exp Bot 62:3765–3779
Li G, Siddiqui H, Teng Y, Lin R, Wan XY et al (2011b) Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat Cell Biol 13:616–622
Liang G, Yu D (2010) Reciprocal regulation among miR395, APS and SULTR2;1 in Arabidopsis thaliana. Plant Signal Behav 5:1257–1259
Liang G, Yang F, Yu D (2010) MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J 62:1046–1057
Lopez-Gomollon S, Mohorianu I, Szittya G, Moulton V, Dalmay T (2012) Diverse correlation patterns between microRNAs and their targets during tomato fruit development indicates different modes of microRNA actions. Planta 236(6):1875–1887
Loudet O, Michael TP, Burger BT, Le Mette C, Mockler TC et al (2008) A zinc knuckle protein that negatively controls morning-specific growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:17193–17198
Lu S, Sun YH, Shi R, Clark C, Li L et al (2005) Novel and mechanical stress-responsive MicroRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 17:2186–2203
Lu S, Sun YH, Chiang VL (2008) Stress-responsive microRNAs in Populus. Plant J 55:131–151
Mahajan S, Pandey GK, Tuteja N (2008) Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Arch Biochem Biophys 471:146–158
Mallory AC, Bartel DP, Bartel B (2005) MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17:1360–1375
Man MZ, Wang X, Wang Y (2000) POWER_SAGE: comparing statistical tests for SAGE experiments. Bioinformatics 16:953–959
Meyers BC, Axtell MJ, Bartel B et al (2008) Criteria for annotation of plant MicroRNAs. Plant Cell 12:3186–3190
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N et al (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W et al (2005a) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Ottow EA, Polle A, Brosche M, Kangasjarvi J, Dibrov P et al (2005b) Molecular characterization of PeNhaD1: the first member of the NhaD Na+/H+ antiporter family of plant origin. Plant Mol Biol 58:75–88
Pantaleo V, Szittya G, Moxon S, Miozzi L, Moulton V et al (2010) Identification of grapevine microRNAs and their targets using high-throughput sequencing and degradome analysis. Plant J 62:960–976
Park J, Kim YS, Kim SG, Jung JH, Woo JC et al (2011) Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol 156:537–549
Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S et al (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA 106:22540–22545
Qin Y, Duan Z, Xia X, Yin W (2011) Expression profiles of precursor and mature microRNAs under dehydration and high salinity shock in Populus euphratica. Plant Cell Rep 30:1893–1907
Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N et al (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199–208
Serrano R, Rodriguez-Navarro A (2001) Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 13:399–404
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M et al (1999) A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11:1743–1754
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136(4):669–687
Wang R, Chen S, Zhou X, Shen X, Deng L et al (2008) Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol 28:947–957
Wu G, Poethig RS (2006) Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133:3539–3547
Wu Y, Ding N, Zhao X, Zhao M, Chang Z et al (2007) Molecular characterization of PeSOS1: the putative Na(+)/H(+) antiporter of Populus euphratica. Plant Mol Biol 65:1–11
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl):S165–S183
Yang Y, Zhang F, Zhao M, An L, Zhang L et al (2007) Properties of plasma membrane H+-ATPase in salt-treated Populus euphratica callus. Plant Cell Rep 26:229–235
Zhang HC, Yin WL, Xia XL (2008) Calcineurin B-Like family in Populus: comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul 56:129–140
Zhu JK (2001a) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Zhu JK (2001b) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu QH, Spriggs A, Matthew L, Fan L, Kennedy G et al (2008) A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res 18:1456–1465
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30972339, 31070597, 30730077), the Ministry of Science and Technology of China (2009CB119101), the Scientific Research and Graduate Training Joint Programs from BMEC (Regulation of Tree WUE) and National Science Foundation (NSF) Plant Genome Program (DBI0922604). B.L. was supported by Peking-Yale Joint Center Monsanto Fellowship, a fellowship from the China Scholarship Council and Beijing Forestry University Technology Innovation Program (BLYJ200902).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, B., Duan, H., Li, J. et al. Global identification of miRNAs and targets in Populus euphratica under salt stress. Plant Mol Biol 81, 525–539 (2013). https://doi.org/10.1007/s11103-013-0010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0010-y