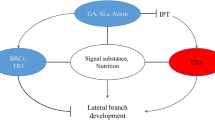
Abstract
Strigolactones have recently been identified as the long sought-after signal required to inhibit shoot branching (Gomez-Roldan et al. 2008; Umehara et al. 2008; reviewed in Dun et al. 2009). Here we briefly describe the evidence for strigolactone inhibition of shoot branching and, more extensively, the broader context of this action. We address the central question of why strigolactone mutants exhibit a varied branching phenotype across a wide range of experimental conditions. Where knowledge is available, we highlight the role of other hormones in dictating these phenotypes and describe those instances where our knowledge of known plant hormones and their interactions falls considerably short of explaining the phenotypes. This review will focus on bud outgrowth in herbaceous species because knowledge on the role of strigolactones in shoot branching to date barely extends beyond this group of plants.

Similar content being viewed by others
References
Adam H, Ouellet F, Kane NA, Agharbaoui Z, Major G, Tominaga Y et al (2007) Overexpression of TaVRN1 in Arabidopsis promotes early flowering and alters development. Plant Cell Physiol 48:1192–1206
Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19:458–472
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M et al (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029
Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44:569–580
Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment and relationship to apical dominance. Planta 194:439–442
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563
Beveridge CA (2000) Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul 32:193–203
Beveridge CA, Murfet IC (1996) The gigas mutant in pea is deficient in the floral stimulus. Physiol Plant 96:637–645
Beveridge CA, Ross JJ, Murfet IC (1992) Mutant dn influences dry matter distribution, assimilate partitioning and flowering in Lathyrus odoratus L. J Exp Bot 43:55–62
Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C (1997) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol 115:1251–1258
Beveridge CA, Batge SL, Ross JJ, Murfet IC (2001) Hormone physiology of pea mutants prevented from flowering by mutations gi or veg1. Physiol Plant 113:285–291
Beveridge CA, Weller JL, Singer SR, Hofer JM (2003) Axillary meristem development. Budding relationships between networks controlling flowering, branching and photoperiod responsiveness. Plant Physiol 131:927–934
Brewer PB, Dun EA, Fergusion BJ, Rameau CA, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150:482–493
Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21:1863–1868
Casal JJ, Fankhauser C, Coupland G, Blázquez MA (2004) Signalling for developmental plasticity. Trends Plant Sci 9:309–314
Cerdán PD, Chory J (2003) Regulation of flowering time by light quality. Nature 423:881–885
Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24:159–169
Cline M (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78:255–266
Cline M (2000) Execution of the auxin replacement apical dominance experiment in temperate woody species. Am J Bot 87:182–190
Cline MG, Riley L (1984) The presentation time for shoot inversion release of apical dominance in Pharbitis nil. Ann Bot 53:897–900
Cline MG, Chatfield SP, Leyser O (2001) NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann Bot 87:61–65
Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA et al (1972) Germination stimulants. II. The structure of strigol—a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94:6198–6199
Davies CR, Wareing PF (1965) Auxin-directed transport of radiophosphorus in stems. Planta 65:139–156
Devitt ML, Stafstrom JP (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29:255–265
Doebley J, Stec A, Hubbard L (1997) The evolution of apical dominance in maize. Nature 386:485–488
Doust AN (2007) Grass architecture: genetic and environmental control of branching. Curr Opin Plant Biol 10:21–25
Doust AN, Devos KM, Gadberry MD, Gale MD, Kellogg EA (2004) Genetic control of branching in foxtail millet. Proc Natl Acad Sci USA 101:9045–9050
Dun EA, Ferguson BJ, Beveridge CA (2006) Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol 142:812–819
Dun EA, Brewer PB, Beveridge CA (2009) Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci 14:364–372
Ferguson BJ, Beveridge CA (2009) Roles for auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol 149:1929–1944
Finlayson S (2007) Arabidopsis TEOSINTE BRANCHED1-LIKE1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol 48:667–677
Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17:464–474
Foo E, Morris SE, Parmenter K, Young N, Wang H, Jones A et al (2007) Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis. Plant Physiol 143:1418–1428
Foucher F, Morin J, Courtiade J, Cadioux S, Ellis N, Banfield MJ, Rameau C (2003) DETERMINATE and LATE FLOWERING are two TERMINAL FLOWER1/CENTRORADIALIS homologs that control two distinct phases of flowering initiation and development in pea. Plant Cell 15:2742–2754
Gomez-Roldan V, Fermas S, Brewer PB, Peuch-Pagès V, Dun EA, Pillot J-P et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Grbić B, Bleecker AB (2000) Axillary meristem development in Arabidopsis thaliana. Plant J 21:215–223
Hayward A, Stirnberg P, Beveridge CA, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Hecht V, Foucher F, Ferrándiz C, Macknight R, Navarro C, Morin J et al (2005) Conservation of Arabidopsis flowering genes in model legumes. Plant Physiol 137:1420–1434
Horvath D (2009) Common mechanisms regulate flowering and dormancy. Plant Sci 177:523–531
Hu W, Zhang S, Zhao Z, Sun C, Zhao Y, Luo D (2003) The analysis of the structure and expression of OsTB1 gene in rice. J Plant Physiol Mol Biol 29:507–514
Humphrey AJ, Beale MH (2006) Strigol: biogenesis and physiological activity. Phytochemistry 67:636–640
Husain SM, Linck AJ (1966) Relationship of apical dominance to the nutrient accumulation in Pisum sativum var. Alaska. Physiol Plant 19:992–1010
Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46:79–86
Jensen CS, Salchert K, Nielsen KK (2001) A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity. Plant Physiol 125:1517–1528
Jiang F, Li C, Jeschke WD, Zhang F (2001) Effect of top excision and replacement by 1-naphthylacetic acid on partition and flow of potassium in tobacco plants. J Exp Bot 52:2143–2150
Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA et al (2006) Branching genes are conserved across species. Genes controlling a novel signal in pea are co-regulated by other long-distance signals. Plant Physiol 142:1014–1026
Kebrom TH, Brutnell TP (2007) The molecular analysis of the shade avoidance syndrome in the grasses has begun. J Exp Bot 58:3079–3089
Kebrom TH, Burson BL, Finlayson SA (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140:1109–1117
King RA, Van Staden J (1988) Differential responses of buds along the shoot of Pisum sativum to isopentyladenine and zeatin application. Plant Physiol Biochem 26:253–259
Kitazawa D, Miyazawa Y, Fujii N, Hoshino A, Iida S, Nitasaka E et al (2008) The gravity-regulated growth of axillary buds is mediated by a mechanism different from decapitation-induced release. Plant Cell Physiol 49:891–900
Klee H (2008) Plant biology: hormones branch out. Nature 455:176–177
Kyozuka J (2007) Control of shoot and root meristem function by cytokinin. Curr Opin Plant Biol 10:442–446
Lang GA, Early JD, Martin GC, Darnell RL (1987) Endo-, para-, and eco-dormancy: physiological terminology and classification for dormancy research. Hortic Sci 22:371–377
Leyser O (2008) Strigolactones and shoot branching: a new trick for a young dog. Dev Cell 15:337–338
Leyser O (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32:694–703
Li CJ, Bangerth F (1999) Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106:415–420
Liew LC, Hecht V, Laurie RE, Knowles CL, Vander Schoor JK, Macknight RC, Weller JL (2009) DIE NEUTRALIS and LATE BLOOMER 1 contribute to regulation of the pea circadian clock. Plant Cell 21:3198–3211
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z et al (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21:1512–1525
López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, Bouwmeester H (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178:863–874
López-Ráez JA, Matusova R, Cardoso C, Jamil M, Charnikhova T, Kohlen W et al (2009) Strigolactones: ecological significance and use as a target for parasitic plant control. Pest Manag Sci 65:471–477
Mader JC, Turnbull CGN, Emery RJN (2003) Transport and metabolism of xylem cytokinins during lateral bud release in decapitated chickpea (Cicer arietinum) seedlings. Physiol Plant 117:118–129
Madoka Y, Mori H (2000) Acropetal disappearance of PsAD1 Protein in pea axillary buds after the release of apical dominance. Plant Cell Physiol 41:556–564
Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934
McSteen P (2009) Hormonal regulation of branching in grasses. Plant Physiol 149:46–55
McSteen P, Leyser O (2005) Shoot branching. Annu Rev Plant Biol 56:353–374
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37:128–138
Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24:55–80
Morris DA (1977) Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.): some implication for polarity and apical dominance. Planta 136:91–96
Morris DA, Johnson CF (1990) The role of auxin efflux carriers in the reversible loss of polar auxin transport in the pea (Pisum sativum L.) stem. Planta 181:117–124
Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138:1665–1672
Murfet IC, Symons GC (2000a) Double mutant rms2 rms5 expresses a transgressive, profuse branching phenotype. Pisum Genet 32:33–38
Murfet IC, Symons GC (2000b) The pea rms2-1 rms4-1 double-mutant phenotype is transgressive. Pisum Genet 32:59–60
Nakagawa M, Shimamoto K, Kyozuka J (2002) Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J 29:743–750
Napoli C (1996) Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol 111:27–37
Napoli CA, Beveridge CA, Snowden KC (1999) Re-evaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr Top Dev Biol 44:127–169
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Nat Acad Sci 101(21):8039–8044
Ongaro V, Leyser O (2008) Hormonal control of shoot branching. J Exp Bot 59:67–74
Ongaro V, Bainbridge K, Williamson L, Leyser O (2008) Interactions between axillary branches of Arabidopsis. Mol Plant 1:388–400
Ouellet F, Overoorde PJ, Theologis A (2001) IAA17/AXR3: biochemical insight into an auxin mutant phenotype. Plant Cell 13:829–841
Phillips IDJ (1968) Nitrogen, phosphorus and potassium distribution in relation to apical dominance in dwarf bean (Phaseolus vulgaris, c.v. Canadian Wonder). J Exp Bot 19:617–627
Pnueli L, Gutfinger T, Hareven D, Ben-Nairn O, Ron N, Adir N et al (2001) Tomato SP-interacting proteins define a conserved signalling system that regulates shoot architecture and flowering. Plant Cell 13:2687–2702
Prasad TK, Cline MG (1985) Shoot inversion-induced ethylene in Pharbitis nil induces the release of apical dominance by restricting shoot elongation. Plant Sci 38:163–172
Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R et al (1998) A common mechanism controls the life cycle and architecture of plants. Development 125:1609–1615
Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5:147–157
Robson PRH, Whitelam GC, Smith H (1993) Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol 102:1179–1184
Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69:361–373
Sachs T, Thimann K (1964) Release of lateral buds from apical dominance. Nature 201:939–940
Sachs T, Thimann KV (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54:136–144
Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y et al (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc Natl Acad Sci USA 106:8392–8397
Shen H, Luong P, Huq E (2007) The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol 145:1471–1483
Shimizu S, Mori H (1998) Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant Cell Physiol 39:255–262
Shimizu-Sato S, Mori H (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127:1405–1413
Shimizu-Sato S, Ike Y, Mori H (2008) PsRBR1 encodes a pea retinoblastoma-related protein that is phosphorylated in axillary buds during dormancy-to-growth transition. Plant Mol Biol 66:125–135
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69:429–435
Snowden KC, Napoli CA (2003) A quantitative study of lateral branching in petunia. Funct Plant Biol 30:987–994
Stafstrom JP (1993) Axillary bud development in pea: apical dominance, growth cycles, hormonal regulation and plant architecture. In: Amasino RM (ed) Cellular communication in plants. Plenum Press, New York, pp 75–86
Stafstrom JP, Sussex IM (1988) Patterns of protein synthesis in dormant and growing vegetative buds of pea. Planta 176:497–505
Stafstrom JP, Sussex IM (1992) Expression of a ribosomal protein gene in axillary buds of pea seedlings. Plant Physiol 100:1494–1502
Stahlberg R, Cosgrove DJ (1992) Rapid alteration in growth rate and electric potentials upon stem excision in pea seedlings. Planta 187:523–531
Stirnberg P, van de Sande K, Leyser HMO (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129:1131–1141
Stirnberg P, Furner IJ, Leyser HMO (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50:80–94
Takeda T, Suwa Y, Suzuki M, Kitano H, Ueguchi-Tanaka M, Ashikari M et al (2003) The OsTB1 gene negatively regulates lateral branching in rice. Plant J 33:513–520
Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K et al (2004) AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol 45:1053–1062
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036
Taylor SA, Murfet IC (1994) A short day mutant in pea is deficient in the floral stimulus. Flower Newsl 18:39–43
Thimann KV, Skoog F (1933) Studies on the growth hormone of plants III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19:714–716
Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substances in Vicia faba. Proc R Soc Lond B Biol Sci 114:317–339
Turnbull CGN, Raymond MAA, Dodd IC, Morris SE (1997) Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during the release of apical dominance. Planta 202:271–276
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Wang G, Römheld V, Li C, Bangerth F (2006) Involvement of auxin and CKs in boron deficiency induced changes in apical dominance of pea plants (Pisum sativum L.). J Plant Physiol 163:591–600
Weberling F (1989) Morphology of flowers and inflorescences. Cambridge University Press, Cambridge
Weller JL, Hecht V, Liew LC, Sussmilch FC, Wenden B, Knowles CL et al (2009) Update on the genetic control of flowering in garden pea. J Exp Bot 60:2493–2499
Woo HE, Chung KM, Park JH, Oh SA, Ahn T, Hong SH et al (2001) ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13:1779–1790
Yan H, Saika H, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M (2007) Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet Syst 82:361–366
Yang HY, Li CJ, Zhang FS (2007) Shoot apex demand determines assimilate and nutrients partitioning and nutrient-uptake rate in tobacco plants. J Integr Plant Biol 49:1654–1661
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225(4):1031–1038
Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W et al (2006) The rice HIGH-TILLERING DWARF1 encoding an orthologue of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48:687–696
Acknowledgments
We thank the Australian Research Council for grant funding and the Australian Postgraduate Award scheme for funding to AH and TW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tanya Waldie and Alice Hayward contributed equally to this work.
Rights and permissions
About this article
Cite this article
Waldie, T., Hayward, A. & Beveridge, C.A. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture?. Plant Mol Biol 73, 27–36 (2010). https://doi.org/10.1007/s11103-010-9599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9599-2