Abstract
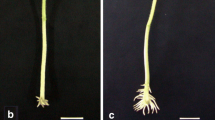
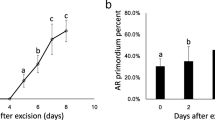
Thirteen α-expansin genes were isolated from Rumex palustris, adding to the six already documented for this species. Five α-expansin genes were selected for expression studies in various organs/tissues of R. palustris, with a focus on roots exposed to aerated or O2-deficient conditions, using real-time RT-PCR. Several cases of differential expression of α-expansin genes in the various root types of R. palustris were documented, and the identity of the dominant transcript differed between root types (i.e., tap root vs. lateral roots vs. adventitious roots). Several genes were expressed differentially in response to low O2. In situ hybridizations showed expansin mRNA expression in the oldest region of the tap root was localized to cells near the vascular cambium; this being the first report of expansin expression associated with secondary growth in roots. In situ hybridization also showed abundant expression of expansin mRNA in the most apical 1 mm of adventitious roots. Such early expression of expansin mRNA in cells soon after being produced by the root apex presumably enables cell wall loosening in the elongation zone of roots. In addition, expression of some expansin mRNAs increased in ‘mature zones’ of roots; these expansins might be involved in root hair formation or in formation of lateral root primordia. The present findings support the notion that large gene families of α-expansins enable flexibility in expression for various organs and tissues as a normal part of plant development, as well as in response to abiotic stress.
Similar content being viewed by others
References
Armstrong, W. 1979. Aeration in higher plants. Adv. Bot. Res. 7: 225–332.
Catala, C., Rose, J. K. C. and Bennett, A. B. 2000. Auxinregulatedgenes encoding cell wall-modifying proteins areexpressed during early tomato fruit growth. Plant Physiol. 122; 527–534.
Cho, H.-T. and Cosgrove, D. J. 2002. Regulation of root hairinitiation and expansin gene expression in arabidopsis. PlantCell 14: 3237–3253.
Cho, H.-T. and Kende, H. 1998. Tissue localization of Expansions in deepwater rice. Plant J. 15: 805–812.
Chomczynski, P. and Sacchi, N. 1987. Single-step method ofRNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 162: 156–159.
Colmer, T. D. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 26: 17–36.
Cosgrove, D. J. 2000a. Expansive growth of plant cell walls. Plant Physiol. Biochem. 38: 109–124.
Cosgrove, D. J. 2000b. Loosening of plant cell walls by Expansions. Nature 407: 321–326.
Cox, M. C. H., Millenaar, F. F., de Jong van Berkel, Y. E. M., Peeters, A. J. M. and Voesenek, L. A. C. J. 2003. Plant movement; submergence-induced petiole elongation in Rumexpalustris depends on hyponastic growth. Plant Physiol 132: 282–291.
Drews, G. N. 1998. In situ hybridization. In: J. M. Martinez-Zapater and J. Salinas (Eds. ), Methods in Molecular Biology Humana Press, Totowa, NJ, USA. 82: pp. 353–371.
Fleming, A. J., McQueen-Mason, S. J., Mandel, T. and Kuhlemeier, C. 1997. Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415–1418.
Feinburg, A. P. and Vogelstein, B. 1984. A technique forradiolabeling DNA restriction endonuclease fragments tohigh specific activity [Addendum], Anal. Biochem. 137: 266–267.
Hutchison, K. W., Singer, P. B., McInnis, S., Diaz-Sala, C. and Greenwood, M. S. 1999. Expansions are conserved in conifersand expressed in hypocotyls in response to exogenous auxin. Plant Physiol. 120: 827–831.
Jackson, M. B. and Drew, M. C. 1984. Effects of flooding ongrowth and metabolism of herbaceous plants. In: T. T. Kozlowski, (Ed. ), Flooding and Plant Growth, Academic Press, New York, pp. 47–128.
Jackson, M. B. and Armstrong, W. 1999. Formation of aerenchymaand the processes of plant ventilation in relation tosoil flooding and submergence. Plant Biol. 1: 274–287.
Justin, S. H. F. W. and Armstrong W. 1987. The anatomicalcharacteristics of roots and plant response to soil flooding. New Phytol. 106: 465–95.
Karnovsky, M. J. 1965. A formaldehyde–glutaraldehyde fixativeof high osmolarity for use in electron microscopy. J. Cell. Biol. 27: 137A–138A.
Kende, H., van der Knaap, E. and Cho, H.-T. 1998. Deep water rice: a model plant to study stem elongation. Plant Physiol. 118: 1105–1110.
Kende, H., Bradford, K. J., Brummell, D. A., Cho, H.-T., Cosgrove, D. J., Fleming, A. J., Gehring, C., Lee, Y., McQueen-Mason, S. J., Rose, J. K. C. and Voesenek L. A. C. J. 2004. Nomenclature for members of the expansin superfamilyof genes and proteins. Plant Mol. Biol. 55: 311–314.
Kiefer, E., Heller, W. and Ernst, D. 2000. A simple and efficientprotocol for isolation of functional RNA from plant tissuesrich in secondary metabolites. Plant Mol. Biol. Rep. 18: 33–39.
Laan, P., Berrevoets, M. J., Lythe, S., Armstrong, W. and Blom, C. W. P. M. 1989. Root morphology and aerenchyma formationas indicators of the flood-tolerance of Rumex species. J. Ecol. 77: 693–703.
Lee, D.-K., Ahn, J. H., Song, S.-K., Choi, Y. D. and Lee, J. S. 2003. Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol. 131: 985–997.
Lee, Y. and Kende, H. 2001. Expression of beta-Expansions is correlated with internodal elongation in deepwater rice. Plant Physiol. 127: 645–654.
Lee, Y., Choi, D. and Kende, H. 2001. Expansions: ever increasing numbers and functions. Curr. Opin. Plant Biol. 4: 527–532.
Lee, Y. and Kende, H. 2002. Expression of alpha-Expansions andexpansin-like genes in deepwater rice. Plant Physiol. 130: 1396–1404.
Li, Y., Darley, C. P., Ongaro, V., Fleming, A., Schipper, O., Baldauf, S. L. and McQueen-Mason, S. J. 2002. Plant Expansions are a complex multigene family with an ancient evoluionary origin. Plant Physiol. 128: 854–864.
Link, B. M. and Cosgrove, D. J. 1998. Acid-growth response anda-expansin in suspension cultures of bright yellow 2 tobacco. Plant Physiol. 118: 907–916.
Livak, K. J., Flood, S. J. A., Marmaro, J., Giusti, W. and Deetz, K. 1995. Oligonucleotides with fluorescent dyes at oppositeends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Meth. Applic 4: 357–362.
Livak, K. J. and Schmittgen, T. D. 2001. Analysis of relativegene expression data using real-time quantitative PCR and the 2) DDCT method. Methods 25: 402–408.
McQueen-Mason, S. J. and Cosgrove, D. J. 1995. Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol. 107: 87–100.
McQueen-Mason, S. J., Durachko, D. M. and Cosgrove, D. J. 1992. Two endogenous proteins that induce cell wallextension in plants. Plant Cell 4: 1425–1433.
Nielsen, H., Engelbrecht, J., Brunak, S. and von Heijne, G. 1997. Identification of prokaryotic and eukaryotic signalpeptides and prediction of their cleavage sites. Prot. Engin10: 1–6.
Peeters, A. J. M., Cox, M. C. H., Benschop, J. J., Vreeburg, R. A. M., Bou, J. and Voesenek L. A. C. J. 2002. Submergenceresearch using Rumex palustris as a model; looking back andgoing forward. J. Exp. Bot. 53: 391–398.
Pien, S., Wyrzykowska, J., McQueen-Mason, S. J., Smart, C. and Fleming, A. 2001. Local expression of expansin inducesthe entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817.
Ponnamperuma, F. N. 1984. Effects of flooding on soils. In: T. T. Kozlowski (Ed. ) Flooding and Plant Growth. AcademicPress, New York, pp. 9–45.
Raskin, I. 1983. A method for measuring leaf volume, density, thickness, and internal gas volume. Hort. Sci. 18: 698–699.
Rose, J. K. C., Cosgrove, D. J., Albersheim, P., Darvill, A. G. and Bennett, A. B. 2000. Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol. 123: 1583–1592.
Sambrook, J., Fritsch, E. F. and Maniatis, T. 1989. MolecularCloning– a laboratory manual,2nd ed Cold Spring Harbor, NY, USA.
Sanger, F., Nicklen, S. and Coulson, A. R. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 74: 5463–5467.
Thomson, C. J., Armstrong, W., Waters, I. and Greenway, H. 1990. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant CellEnviron. 13: 395–403.
Vartapetian, B. B. and Jackson, M. B. 1997. Plant adaptations to anaerobic stress. Ann. Bot. 79: 3–20.
Visser, E. J. W., Blom, C. W. P. M. and Voesenek, L. A. C. J. 1996a. Flooding-induced adventitious root formation in Rumex: morphology and development in an ecological perspective. Acta Bot. Neerl. 45: 17–28.
Visser, E. J. W., Cohen, J. D., Barendse, G. W. M., Blom, C. W. P. M. and Voesenek, L. A. C. J. 1996b. An ethylenemediatedincrease in sensitivity to auxin induces adventitiousroot formation in flooded Rumex palustris Sm. Plant Physiol. 112: 1687–1692.
Visser, E. J. W., Colmer, T. D., Blom, C. W. P. M. and Voesenek, L. A. C. J. 2000. Changes in growth, porosity, and radialoxygen loss from adventitious roots of selected mono-anddicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ. 23: 1237–1245.
Voesenek, L. A. C. J., Benschop, J. J., Bou, J., Cox, M. C. H., Groeneveld, H. W., Millenaar, F. F., Vreeburg, R. A. M. and Peeters, A. J. M. 2003. Interactions between plant hormonesregulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann. Bot. 91: 205–211.
Voesenek, L. A. C. J., Perik, P. J. M., Blom, C. W. P. M. and Sassen, M. M. A. 1990. Petiole elongation in Rumex duringsubmergence and ethylene exposure: the relative contributionsof cell division and cell expansion. J Plant Growth Reg. 9: 13–17.
Voesenek, L. A. C. J., Rijnders, J. H. G. M., Peeters, A. J. M., vander Steeg, H. M. and de Kroon, H. 2004. Plant hormonesregulate fast shoot elongation under water; from genes to communities. Ecology 85: 16–27.
Vreeburg, R. A. M. 2004. Expansions in submergence-inducedpetiole elongation of Rumex palustris: kinetics and regulation. Ph. D. Dissertation, Utrecht University, Utrecht, TheNetherlands.
Vriezen, W. H., De Graaf, B., Mariani, C. and Voesenek, L. A. C. J. 2000. Submergence induces Expansin gene expressionin flooding–tolerant Rumex palustris and not in flooding–intolerant R. acetosa. Planta 210: 956–963.
Wiengweera, A., Greenway, H. and Thomson, C. J. 1997. The use of agar nutrient solution to simulate lack of convectionin waterlogged soils. Ann. Bot. 80: 115–123.
Wu, Y., Meeley, R. B. and Cosgrove, D. J. 2001a. Analysis andexpression of the alpha-expansin and beta-expansin genefamilies in maize. Plant Physiol. 126: 222–232.
Wu, Y., Thorne, E. T., Sharp, R. E. and Cosgrove, D. J. 2001b. Modification of expansin transcript levels in the maizeprimary root at low water potentials. Plant Physiol 126: 1471–1479.
Zhang, N. and Hasenstein, K. H. 2000. Distribution of Expansionsin graviresponding maize roots. Plant Cell Physiol. 41: 1305–1312.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Colmer, T.D., Peeters, A.J.M., Wagemaker, C.A.M. et al. Expression of α-expansin genes during root acclimations to O2 deficiency in Rumex palustris . Plant Mol Biol 56, 423–437 (2004). https://doi.org/10.1007/s11103-004-3844-5
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-3844-5