Abstract
Purpose
Oxidized interferons have been shown to aggregate and cause immunogenicity. In this study, the structural mechanisms underlying oxidation-induced interferon alpha-2a (IFNA2a) aggregation and loss of function were examined.
Methods
IFNA2a was oxidized using 0.037% vol/vol hydrogen peroxide. Oxidized protein was probed using biophysical methods that include denaturant melts, particle counting, proteolysis-coupled mass spectrometry, and 2D NMR.
Results
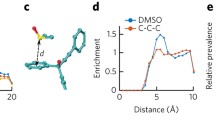
Oxidized IFNA2a did not show major changes in its secondary structure, but showed minor changes in tertiary structure when compared to the unoxidized protein. In addition, a significant loss of conformational stability was observed upon oxidation. Correspondingly, increased protein aggregation was observed resulting in the formation of sub-visible particles. Oxidized protein showed decreased biological function in terms of its anti-viral potency and cytopathic inhibition efficacy. Proteolysis-coupled mass spectrometry identified five methionine residues that were oxidized with no correlation between the extent of oxidation and their accessible surface area. 2D 15N-1H HSQC NMR identified residue-level local structural changes in the protein upon oxidation, which were not detectable by global probes such as far-UV circular dichroism and fluorescence.
Conclusions
Increased protein aggregation and decreased function of IFNA2a upon oxidation correlated with the site of modification identified by proteolysis-coupled mass spectrometry and local structural changes in the protein detected by 2D NMR.








Similar content being viewed by others
Abbreviations
- ASA:
-
Accessible surface area
- CD:
-
Circular dichroism
- GdmCl:
-
Guanidinium chloride
- H2O2 :
-
Hydrogen peroxide
- IFNA2a:
-
Interferon alpha-2a
- MRE:
-
Mean residue ellipticity
- MS:
-
Mass spectrometry
- NMR:
-
Nuclear magnetic resonance.
References
Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185(2):129–88.
Manning MC, Liu J, Li T, Holcomb RE. Rational design of liquid formulations of proteins. Adv Protein Chem Struct Biol. 2018;112:1–59.
Torosantucci R, Schoneich C, Jiskoot W. Oxidation of therapeutic proteins and peptides: structural and biological consequences. Pharm Res. 2014;31(3):541–53.
Wakankar AA, Borchardt RT. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J Pharm Sci. 2006;95(11):2321–36.
Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10(4):307–77.
Shah DD, Zhang J, Hsieh M-C, Sundaram S, Maity H, Mallela KMG. Effect of peroxide- versus alkoxyl-induced chemical oxidation on the structure, stability, aggregation, and function of a therapeutic monoclonal antibody. J Pharm Sci. 2018. https://doi.org/10.1016/j.xphs.2018.07.024.
Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208.
Li S, Schoneich C, Borchardt RT. Chemical instability of protein pharmaceuticals: mechanisms of oxidation and strategies for stabilization. Biotechnol Bioeng. 1995;48(5):490–500.
Chu JW, Yin J, Brooks BR, Wang DI, Ricci MS, Brems DN, et al. A comprehensive picture of non-site specific oxidation of methionine residues by peroxides in protein pharmaceuticals. J Pharm Sci. 2004;93(12):3096–102.
Mason BD, Schoneich C, Kerwin BA. Effect of pH and light on aggregation and conformation of an IgG1 mAb. Mol Pharm. 2012;9(4):774–90.
Schoneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer's disease. Biochim Biophys Acta. 2005;1703(2):111–9.
Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97(8):2924–35.
Kerwin BA, Remmele RL, Jr. Protect from light: Photodegradation and protein biologics. J Pharm Sci 2007;96(6):1468–1479.
Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75.
Zbacnik TJ, Holcomb RE, Katayama DS, Murphy BM, Payne RW, Coccaro RC, et al. Role of buffers in protein formulations. J Pharm Sci. 2017;106(3):713–33.
Kishore RS, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, et al. Degradation of polysorbates 20 and 80: studies on thermal autoxidation and hydrolysis. J Pharm Sci. 2011;100(2):721–31.
Shah DD, Zhang J, Maity H, Mallela KMG. Effect of photo-degradation on the structure, stability, aggregation, and function of an IgG1 monoclonal antibody. Int J Pharm. 2018;547(1–2):438–49.
WHO. Model list of essential medicines. http://www.hoint/medicines/publications/essentialmedicines/en/. 2017.
Abdolvahab MH, Fazeli A, Halim A, Sediq AS, Fazeli MR, Schellekens H. Immunogenicity of recombinant human interferon beta-1b in immune-tolerant transgenic mice corresponds with the biophysical characteristics of aggregates. J Interf Cytokine Res. 2016;36(4):247–57.
Barnard JG, Babcock K, Carpenter JF. Characterization and quantitation of aggregates and particles in interferon-beta products: potential links between product quality attributes and immunogenicity. J Pharm Sci. 2013;102(3):915–28.
Hochuli E. Interferon immunogenicity: technical evaluation of interferon-alpha 2a. J Interf Cytokine Res. 1997;17(Suppl 1):S15–21.
Ryff JC. Clinical investigation of the immunogenicity of interferon-alpha 2a. J Interf Cytokine Res. 1997;17(Suppl 1):S29–33.
Nazarov VD, Lapin SV, Mazing AV, Evdoshenko EP, Totolian AA. Immunogenicity of human interferon-beta-containing pharmaceuticals. Biochemistry (Mosc). 2016;81(11):1396–400.
Chou DK, Krishnamurthy R, Manning MC, Randolph TW, Carpenter JF. Effects of solution conditions on methionine oxidation in albinterferon alfa-2b and the role of oxidation in its conformation and aggregation. J Pharm Sci. 2013;102(2):660–73.
van Beers MM, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm Res. 2011;28(10):2393–402.
Hermeling S, Schellekens H, Maas C, Gebbink MF, Crommelin DJ, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci. 2006;95(5):1084–96.
Torosantucci R, Sharov VS, van Beers M, Brinks V, Schöneich C, Jiskoot W. Identification of oxidation sites and covalent cross-links in metal catalyzed oxidized interferon beta-1a: potential implications for protein aggregation and immunogenicity. Mol Pharm. 2013;10(6):2311–22.
Hermeling S, Aranha L, Damen JM, Slijper M, Schellekens H, Crommelin DJ, et al. Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon alpha2b. Pharm Res. 2005;22(12):1997–2006.
Cheng W, Zheng X, Yang M. Hydrogen peroxide induced protein oxidation during storage and lyophilization process. J Pharm Sci. 2016;105(6):1837–42.
Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci. 2002;91(10):2252–64.
Jaeger J, Sorensen K, Wolff SP. Peroxide accumulation in detergents. J Biochem Biophys Methods. 1994;29(1):77–81.
Maggio ET. Polysorbates, peroxides, protein aggregation, and immunogenicity: a growing concern. J Excip Food Chem. 2012;3(2):45–53.
Yin J, Chu JW, Ricci MS, Brems DN, Wang DI, Trout BL. Effects of excipients on the hydrogen peroxide-induced oxidation of methionine residues in granulocyte colony-stimulating factor. Pharm Res. 2005;22(1):141–7.
Ji JA, Zhang B, Cheng W, Wang YJ. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: mechanisms and stabilization. J Pharm Sci. 2009;98(12):4485–500.
Bis RL, Stauffer TM, Singh SM, Lavoie TB, Mallela KM. High yield soluble bacterial expression and streamlined purification of recombinant human interferon alpha-2a. Protein Expr Purif. 2014;99:138–46.
Thirumangalathu R, Krishnan S, Bondarenko P, Speed-Ricci M, Randolph TW, Carpenter JF, et al. Oxidation of methionine residues in recombinant human interleukin-1 receptor antagonist: implications of conformational stability on protein oxidation kinetics. Biochemistry. 2007;46(21):6213–24.
Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solutions in the UV). Anal Biochem. 1972;49(2):474–8.
Barnett GV, Balakrishnan G, Chennamsetty N, Meengs B, Meyer J, Bongers J, et al. Enhanced precision of circular dichroism spectral measurements permits detection of subtle higher order structural changes in therapeutic proteins. J Pharm Sci. 2018;107(10):2559–69.
Pace CN. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Meth Enzymol. 1986;131:266–80.
Santoro MM, Bolen DW. A test of the linear extrapolation of unfolding free energy changes over an extended denaturant concentration range. Biochemistry. 1992;31(20):4901–7.
Santoro MM, Bolen DW. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha-chymotrypsin using different denaturants. Biochemistry. 1988;27(21):8063–8.
Pace CN, Shaw KL. Linear extrapolation method of analyzing solvent denaturation curves. Proteins: Struct Funct Bioinform. 2000;41(S4):1–7.
Kalonia C, Kumru OS, Prajapati I, Mathaes R, Engert J, Zhou S, et al. Calculating the mass of subvisible protein particles with improved accuracy using microflow imaging data. J Pharm Sci. 2015;104(2):536–47.
Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ, Rance M. Protein NMR spectroscopy: principles and practice: Elsevier science; 2010.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual: cold spring harbor laboratory press; 1989.
Bis RL, Singh SM, Cabello-Villegas J, Mallela KMG. Role of benzyl alcohol in the unfolding and aggregation of interferon alpha-2a. J Pharm Sci. 2015;104(2):407–15.
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6(3):277–93.
Klaus W, Gsell B, Labhardt AM, Wipf B, Senn H. The three-dimensional high resolution structure of human interferon alpha-2a determined by heteronuclear NMR spectroscopy in solution. J Mol Biol. 1997;274(4):661–75.
Panjwani N, Hodgson DJ, Sauve S, Aubin Y. Assessment of the effects of pH, formulation and deformulation on the conformation of interferon alpha-2 by NMR. J Pharm Sci. 2010;99(8):3334–42.
Lavoie TB, Kalie E, Crisafulli-Cabatu S, Abramovich R, DiGioia G, Moolchan K, et al. Binding and activity of all human alpha interferon subtypes. Cytokine. 2011;56(2):282–9.
Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016(4). https://doi.org/10.1101/pdb.prot087379.
Dunigan DD, Waters SB, Owen TC. Aqueous soluble tetrazolium/formazan MTS as an indicator of NADH- and NADPH-dependent dehydrogenase activity. BioTechniques. 1995;19(4):640–9.
Leblanc Y, Ramon C, Bihoreau N, Chevreux G. Charge variants characterization of a monoclonal antibody by ion exchange chromatography coupled on-line to native mass spectrometry: case study after a long-term storage at +5 degrees C. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1048:130–9.
Saunders CC, Stites WE. An electrophoretic mobility shift assay for methionine sulfoxide in proteins. Anal Biochem. 2012;421(2):767–9.
Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1(6):2876–90.
Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751(2):119–39.
Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer Science; 2006.
Pace CN. Measuring and increasing protein stability. Trends Biotechnol. 1990;8(4):93–8.
Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4(10):2138–48.
Scholtz JM, Grimsley GR, Pace CN. Solvent denaturation of proteins and interpretations of the m value. Meth Enzymol. 2009;466:549–65.
Piehler J, Thomas C, Garcia KC, Schreiber G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol Rev. 2012;250(1):317–34.
Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22(4):243–51.
Kalie E, Jaitin DA, Podoplelova Y, Piehler J, Schreiber G. The stability of the ternary interferon-receptor complex rather than the affinity to the individual subunits dictates differential biological activities. J Biol Chem. 2008;283(47):32925–36.
Wishart DS. Characterization of biopharmaceuticals by NMR spectroscopy. Trends Anal Chem. 2013;48:96–111.
Nguyen TH. Oxidation degradation of protein pharmaceuticals. In. Formulation and delivery of proteins and peptides: American Chemical Society; 1994. p. 59–71.
Q1A(R2). Stability testing of new drug substances and products. Federal Registry. 2003;68(225):65717–8.
Q2(R1). Validation of analytical procedures: Text and methodology. Federal Registry. 1995;60:11260.
Hui A, Lam XM, Kuehl C, Grauschopf U, Wang YJ. Kinetic modeling of methionine oxidation in monoclonal antibodies from hydrogen peroxide spiking studies. PDA J Pharm Sci Technol. 2015;69(4):511–25.
Knepp VM, Whatley JL, Muchnik A, Calderwood TS. Identification of antioxidants for prevention of peroxide-mediated oxidation of recombinant human ciliary neurotrophic factor and recombinant human nerve growth factor. PDA J Pharm Sci Technol. 1996;50(3):163–71.
Wang Y, Li X, Liu YH, Richardson D, Li H, Shameem M, et al. Simultaneous monitoring of oxidation, deamidation, isomerization, and glycosylation of monoclonal antibodies by liquid chromatography-mass spectrometry method with ultrafast tryptic digestion. MAbs. 2016;8(8):1477–86.
Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18(2):424–33.
Masato A, Kiichi F, Uchiyama S. Suppression of methionine oxidation of a pharmaceutical antibody stored in a polymer-based syringe. J Pharm Sci. 2016;105(2):623–9.
Folzer E, Diepold K, Bomans K, Finkler C, Schmidt R, Bulau P, et al. Selective oxidation of methionine and tryptophan residues in a therapeutic IgG1 molecule. J Pharm Sci. 2015;104(9):2824–31.
Gitlin G, Tsarbopoulos A, Patel ST, Sydor W, Pramanik BN, Jacobs S, et al. Isolation and characterization of a monomethioninesulfoxide variant of interferon alpha-2b. Pharm Res. 1996;13(5):762–9.
Chu JW, Brooks BR, Trout BL. Oxidation of methionine residues in aqueous solutions: free methionine and methionine in granulocyte colony-stimulating factor. J Am Chem Soc. 2004;126(50):16601–7.
Bobrowski K, Hug GL, Pogocki D, Marciniak B, Schoneich C. Stabilization of sulfide radical cations through complexation with the peptide bond: mechanisms relevant to oxidation of proteins containing multiple methionine residues. J Phys Chem B. 2007;111(32):9608–20.
Pan B, Abel J, Ricci MS, Brems DN, Wang DI, Trout BL. Comparative oxidation studies of methionine residues reflect a structural effect on chemical kinetics in rhG-CSF. Biochemistry. 2006;45(51):15430–43.
Chennamsetty N, Quan Y, Nashine V, Sadineni V, Lyngberg O, Krystek S. Modeling the oxidation of methionine residues by peroxides in proteins. J Pharm Sci. 2015;104(4):1246–55.
Agrawal NJ, Dykstra A, Yang J, Yue H, Nguyen X, Kolvenbach C, et al. Prediction of the hydrogen peroxide-induced methionine oxidation propensity in monoclonal antibodies. J Pharm Sci. 2018;107(5):1282–9.
Soenderkaer S, Carpenter JF, van de Weert M, Hansen LL, Flink J, Frokjaer S. Effects of sucrose on rFVIIa aggregation and methionine oxidation. Eur J Pharm Sci. 2004;21(5):597–606.
Mulinacci F, Poirier E, Capelle MA, Gurny R, Arvinte T. Influence of methionine oxidation on the aggregation of recombinant human growth hormone. Eur J Pharm Biopharm. 2013;85(1):42–52.
DePaz RA, Barnett CC, Dale DA, Carpenter JF, Gaertner AL, Randolph TW. The excluding effects of sucrose on a protein chemical degradation pathway: methionine oxidation in subtilisin. Arch Biochem Biophys. 2000;384(1):123–32.
Chill JH, Quadt SR, Levy R, Schreiber G, Anglister J. The human type I interferon receptor: NMR structure reveals the molecular basis of ligand binding. Structure. 2003;11(7):791–802.
Liu D, Ren D, Huang H, Dankberg J, Rosenfeld R, Cocco MJ, Li L, Brems DN, Remmele RL, Jr. Structure and stability changes of human IgG1 Fc as a consequence of methionine oxidation. Biochemistry 2008;47(18):5088–5100.
Wang S, Ionescu R, Peekhaus N, Leung JY, Ha S, Vlasak J. Separation of post-translational modifications in monoclonal antibodies by exploiting subtle conformational changes under mildly acidic conditions. J Chromato A. 2010;1217(42):6496–502.
Burkitt W, Domann P, O'Connor G. Conformational changes in oxidatively stressed monoclonal antibodies studied by hydrogen exchange mass spectrometry. Protein Sci. 2010;19(4):826–35.
Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9(8):1716–28.
Luo Q, Joubert MK, Stevenson R, Ketchem RR, Narhi LO, Wypych J. Chemical modifications in therapeutic protein aggregates generated under different stress conditions. J Biol Chem. 2011;286(28):25134–44.
Steinmann D, Ji JA, Wang YJ, Schoneich C. Oxidation of human growth hormone by oxygen-centered radicals: formation of leu-101 hydroperoxide and tyr-103 oxidation products. Mol Pharm. 2012;9(4):803–14.
Brinson RG, Ghasriani H, Hodgson DJ, Adams KM, McEwen I, Freedberg DI, et al. Application of 2D-NMR with room temperature NMR probes for the assessment of the higher order structure of filgrastim. J Pharm Biomed Anal. 2017;141:229–33.
Aubin Y, Hodgson DJ, Thach WB, Gingras G, Sauve S. Monitoring effects of excipients, formulation parameters and mutations on the high order structure of filgrastim by NMR. Pharm Res. 2015;32(10):3365–75.
Arbogast LW, Delaglio F, Schiel JE, Marino JP. Multivariate analysis of two-dimensional (1)H, (13)C methyl NMR spectra of monoclonal antibody therapeutics to facilitate assessment of higher order structure. Anal Chem. 2017;89(21):11839–45.
Arbogast LW, Brinson RG, Marino JP. Mapping monoclonal antibody structure by 2D 13C NMR at natural abundance. Anal Chem. 2015;87(7):3556–61.
Singh SM, Bandi S, Jones DNM, Mallela KMG. Effect of polysorbate 20 and polysorbate 80 on the higher-order structure of a monoclonal antibody and its Fab and Fc fragments probed using 2D nuclear magnetic resonance spectroscopy. J Pharm Sci. 2017;106(12):3486–98.
Amezcua CA, Szabo CM. Assessment of higher order structure comparability in therapeutic proteins using nuclear magnetic resonance spectroscopy. J Pharm Sci. 2013;102(6):1724–33.
Japelj B, Ilc G, Marusic J, Sencar J, Kuzman D, Plavec J. Biosimilar structural comparability assessment by NMR: from small proteins to monoclonal antibodies. Sci Rep. 2016;6:32201.
Ghasriani H, Hodgson DJ, Brinson RG, McEwen I, Buhse LF, Kozlowski S, et al. Precision and robustness of 2D-NMR for structure assessment of filgrastim biosimilars. Nature Biotech. 2016;34(2):139–41.
Poppe L, Jordan JB, Rogers G, Schnier PD. On the analytical superiority of 1D NMR for fingerprinting the higher order structure of protein therapeutics compared to multidimensional NMR methods. Anal Chem. 2015;87(11):5539–45.
Hodgson DJ, Aubin Y. Assessment of the structure of pegylated-recombinant protein therapeutics by the NMR fingerprint assay. J Pharm Biomed Anal. 2017;138:351–6.
Franks J, Glushka JN, Jones MT, Live DH, Zou Q, Prestegard JH. Spin diffusion editing for structural fingerprints of therapeutic antibodies. Anal Chem. 2016;88(2):1320–7.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Figure S1
Aggregation propensity predictions for IFNA2a by various computational programs. Black rectangles indicate overlapping regions that were predicted to be aggregation hot-spots by all the three programs. (PNG 270 kb)
Rights and permissions
About this article
Cite this article
Shah, D.D., Singh, S.M. & Mallela, K.M.G. Effect of Chemical Oxidation on the Higher Order Structure, Stability, Aggregation, and Biological Function of Interferon Alpha-2a: Role of Local Structural Changes Detected by 2D NMR. Pharm Res 35, 232 (2018). https://doi.org/10.1007/s11095-018-2518-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2518-y