Abstract
Purpose
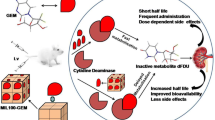
Despite being widely used for the treatment of several solid tumors, Gemcitabine (GEM) exhibits several suboptimal pharmacokinetic properties. Therefore, the design of nanoparticle delivery systems is a promising strategy to enhance GEM pharmacokinetic properties.
Methods
In this work, the polymeric material methoxy poly(ethylene glycol)-block-poly(L-glutamic acid)-graft-gemcitabine (mPEG-b-PLG-g-GEM) was synthesized through the covalent conjugation of GEM with the carboxylic group of methoxy poly(ethylene glycol)-block-poly (L-glutamic acid) (mPEG-b-PLG) (mPEG113, Mn = 5000). mPEG-PLG-GEM/CaP nanoparticles were prepared through the simple mixing of calcium and phosphate/mPEG-PLG-GEM solutions. mPEG-PLG-GEM was embedded in the calcium phophate (CaP) backbone via electrostatic interactions.
Results
After incubation in plasma at 37°C for 24 h, gemcitabine was degraded by 24.6% for the mPEG-PLG-GEM, 14.7% for the mPEG-PLG-GEM/CaP nanoparticles, and 90% for the free gemcitabine solution. It was observed that mPEG-PLG-GEM and mPEG-PLG-GEM/CaP improved the area-under-curve (AUC) values by 5.26-fold and 6.33-fold compared to free drug, respectively.
Conclusion
The amide bond linked gemcitabine polymers was able to protect GEM from cytidine deaminase degradation in vivo, and the skeleton formed by the calcium phosphate enhanced the stability and prolonged the half-life of GEM. Importantly, mPEG-PLG-GEM/CaP nanoparticles elevated the GEM plasma concentration in an animal model.









Similar content being viewed by others
Abbreviations
- CDA:
-
Cytidine deaminase
- dCK:
-
Deoxycytidine kinase
- dCTD:
-
Deoxycytidylate deaminase
- dFdC:
-
Difluorodeoxycytidine
- dFdCTP:
-
Deoxycytidine 5′-triphosphate
- dFdU:
-
Diflurodeoxyuridine
- dFdUMP:
-
Di-fluorodeoxyuridine monophosphate
- DLC:
-
Drug loading content
- DLS:
-
Dynamic laser light scattering
- EDS:
-
Energy Dispersive Spectroscopy
- FT-IR:
-
Fourier transform infrared spectroscopy
- HPLC:
-
High Performance Liquid Chromatography
- IR:
-
Infrared Radiation
- LC / MS:
-
Liquid chromatography-mass spectrometry
- PK:
-
Pharmacokinetics
- PLG-g-mPEG:
-
Poly(Lglutamic acid)-g-methoxy poly(ethylene glycol) copolymer
- PBS:
-
Phosphate buffered saline
- TEM:
-
Transmission electron microscopy
- UV-Vis:
-
Ultra-violet-visible
- XRD:
-
X-ray Diffraction
References
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–72.
Xu H, Paxton JW, Wu Z. Development of long-circulating pH-sensitive liposomes to circumvent gemcitabine resistance in pancreatic cancer cells. Pharm Res. 2016;33(7):1628–37.
Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 1994;12(9):1821–6.
Li M, Li H, Cheng X, Wang X, Li L, Zhou T, et al. Preclinical pharmacokinetic/pharmacodynamic models to predict schedule-dependent interaction between erlotinib and gemcitabine. Pharm Res. 2013;30(5):1400–8.
Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, et al. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 2012;13(11):2731–6.
Stadler WM, Kuzel T, Roth B, Raghavan D, Dorr FA. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1997;15(11):3394–8.
Dyawanapelly S, Kumar A, Chourasia MK. Lessons learned from gemcitabine: impact of therapeutic carrier systems and gemcitabine’s drug conjugates on cancer therapy. Crit Rev Ther Drug Carrier Syst. 2017;34(1):63–96.
Ma D, Wang J, Hao X, Wang Y, Hu X, Xing P, et al. 70PA retrospective analysis to explore the value of gemcitabine combined with cisplatin as adjuvant chemotherapy of NSCLC. Ann Oncol. 2017;28(suppl_2).
Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, Lluch A, Llombart A, du Bois A, Kreienberg R, et al. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol. 2009;21(1):1753–1760.
Ejb D, Adr H, Rosing H, Jhm S, Beijnen JH. Intracellular pharmacokinetics of gemcitabine, its deaminated metabolite 2′,2′-difluorodeoxyuridine and their nucleotides. Br J Clin Pharmacol. 2018.
Yong WP. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev. 2009;41(2):77.
Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res. 1992;52(3):533–9.
Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(suppl_5):v7–v12.
Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2′,2′-difluorodeoxycytidine (gemcitabine) administration in leukemia. Cancer Res. 1990;50(21):6823–6.
Dubey RD, Saneja A, Gupta PK, Gupta PN. Recent advances in drug delivery strategies for improved therapeutic efficacy of gemcitabine. Eur J Pharm Sci. 2016;93:147–62.
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9(3):491–8.
Haperen VWTRV, Veerman G, Boven E, Noordhuis P, Vermorken JB, Peters GJ. Schedule dependence of sensitivity to 2′,2′-difluorodeoxycytidine (gemcitabine) in relation to accumulation and retention of its triphosphate in solid tumour cell lines and solid tumours. Biochem Pharmacol. 1994;48(7):1327–39.
Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine). Drug Resist Updat. 2002;5(1):19–33.
Caloro M, Lionetto L, Cuomo I, Simonetti A, Pucci D, Persis SD, et al. An improved simple LC–MS/MS method for the measurement of serum aripiprazole and its major metabolite. J Pharm Biomed Anal. 2012;62(6):135–9.
Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142(3):416–21.
And YK, Kataoka K. Block copolymer self-assembly into monodispersive nanoparticles with hybrid core of antisense DNA and calcium phosphate. Langmuir. 2015;18(12):4539–43.
Zhang Y, Kim WY, Huang L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials. 2013;34(13):3447–58.
Sloat BR, Sandoval MA, Li D, Chung WG, DSP L-P, Proteau PJ, et al. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int J Pharm. 2011;409(1):278–88.
Wang WW, Li C, Zhang J, Dong AJ, Kong DL. Tailor-made gemcitabine prodrug nanoparticles from well-defined drug-polymer amphiphiles prepared by controlled living radical polymerization for cancer chemotherapy. J Mater Chem B. 2014;2(13):1891–901.
Vandana M, Sahoo SK. Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials. 2010;31(35):9340–56.
Zhu S, Lansakara-P DS, Li X, Cui Z. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjug Chem. 2012;23(5):966–80.
Cavallaro G, Licciardi M, Salmaso S, Caliceti P, Gaetano G. Folate-mediated targeting of polymeric conjugates of gemcitabine. Int J Pharm. 2006;307(2):258–69.
Yu H, Tang Z, Li M, Song W, Zhang D, Zhang Y, et al. Cisplatin loaded poly (L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for potential cancer therapy: preparation, in vitro and in vivo evaluation. J Biomed Nanotechnol. 2016;12(1):69–78.
Yu H, Tang Z, Zhang D, Song W, Zhang Y, Yang Y, et al. Pharmacokinetics, biodistribution and in vivo efficacy of cisplatin loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for tumor therapy. J Control Release. 2015;205:89–97.
Warnant J, Marcotte N, Reboul J, Layrac G, Aqil A, Jerôme C, et al. Physicochemical properties of pH-controlled polyion complex (PIC) micelles of poly(acrylic acid)-based double hydrophilic block copolymers and various polyamines. Anal Bioanal Chem. 2012;403(5):1395–404.
Wu C, You J, Wang X. Thermal decomposition mechanism and kinetics of gemcitabine. J Anal Appl Pyrolysis. 2018;130:118–26.
Ravindran S, Suthar JK, Rokade R, Deshpande P, Singh P, Pratinidhi A, et al. Pharmacokinetics, metabolism, distribution and permeability of nanomedicine. Curr Drug Metab. 2018;19:327–34.
Warnecke A, Fichtner I, Sass G, Kratz F. Synthesis, cleavage profile, and antitumor efficacy of an albumin-binding prodrug of methotrexate that is cleaved by plasmin and cathepsin B. Arch Pharm. 2010;340(8):389–95.
Yang Z, Ma M, Xu B. Using matrix metalloprotease-9 (MMP-9) to trigger supramolecular hydrogelation. Soft Matter. 2009;5(13):2546–8.
Koda D, Maruyama T, Minakuchi N, Nakashima K, Goto M. Proteinase-mediated drastic morphological change of peptide-amphiphile to induce supramolecular hydrogelation. Chem Commun. 2010;46(6):979–81.
Jin S, Wan J, Meng L, Huang X, Guo J, Liu L, et al. Biodegradation and toxicity of protease/redox/pH stimuli-responsive PEGlated PMAA nanohydrogels for targeting drug delivery. ACS Appl Mater Interfaces. 2015;7(35):19843–52.
ACKNOWLEDGMENTS AND DISCLOSURES
We sincerely thank Amanda Pearce for the linguistic assistance during the revision of this manuscript.
Funding
This work was supported by the Financial Grant from China Postdoctoral Science Foundation (2016 M600216) and Foundation of Shenyang Pharmaceutical University (ZQN2016008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, W., Tian, P., Ding, N. et al. Improving Plasma Stability and Bioavailability In Vivo of Gemcitabine Via Nanoparticles of mPEG-PLG-GEM Complexed with Calcium Phosphate. Pharm Res 35, 230 (2018). https://doi.org/10.1007/s11095-018-2506-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2506-2