ABSTRACT
Purpose
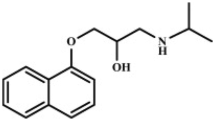
The peptide hormone glucagon, used to treat hypoglycaemic incidents, is prone to aggregation. Generating alternatives with better stability is of pharmaceutical interest in the treatment of diabetes. Here we investigate the impact of six different surfactants on the solubility and stability of ZP-GA-1, a stable version of glucagon.
Methods
We use chemical surfactants (sodium dodecyl sulphate, dodecyl maltoside and polysorbate 20) and the biosurfactants rhamnolipid, sophorolipid and surfactin. We investigate their interaction with ZP-GA-1 by pyrene fluorescence, circular dichroism and isothermal titration calorimetry.
Results
All six surfactants induce α-helical structure in ZP-GA-1, SDS having the biggest impact and polysorbate 20 the smallest. SDS keeps ZP-GA-1 solubilised over >48 days as opposed to 29 days in DDM, 3 days in polysorbate 20 and 0 days in buffer. Similarly, much less SDS than DDM, polysorbate 20 or biosurfactant is needed to redissolve aggregated ZP-GA-1. ITC confirms this trend, with SDS exhibiting very strong, and polysorbate 20 very weak interactions.
Conclusion
Simple surfactant structures promote stronger peptide interactions. ITC shows promise as a general strategy to predict surfactants’ solubilising powers. Stronger enthalpic interactions improved the absolute solubility of ZP-GA-1 and their strength correlated to the absolute solubility of the peptides though not to the kinetics of precipitation.






Similar content being viewed by others
Abbreviations
- DDM:
-
Dodecyl maltoside
- Ps20:
-
Polysorbate 20
- RL:
-
Rhamnolipid
- SDS:
-
Sodium dodecyl sulphate
- SF:
-
Surfactin
- SL:
-
Sophorolipid
- ZP-GA-1:
-
Glucagon analogue
References
Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, et al. The human glucagon receptor encoding gene: structure, cDNA sequence and chromosomal localization. Gene. 1994;140(2):203–9.
Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3):1039–48.
Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes, Metab Syndr Obes: Targets Ther. 2011;4:337–46.
Blauw H, Wendl I, DeVries JH, Heise T, Jax T. Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes Obes Metab. 2015.
Russell SJ, Hillard MA, Balliro C, Magyar KL, Selagamsetty R, Sinha M, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–43.
Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35(11):2148–55.
Onoue S, Yamamoto K, Kawabata Y, Hirose M, Mizumoto T, Yamada S. Novel dry powder inhaler formulation of glucagon with addition of citric acid for enhanced pulmonary delivery. Int J Pharm. 2009;382(1–2):144–50.
Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2006;355(3):501–23.
Beaven GH, Gratzer WB, Davies HG. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969;11(1):37–42.
Onoue S, Ohshima K, Debari K, Koh K, Shioda S, Iwasa S, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21(7):1274–83.
Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MF. A role for protein misfolding in immunogenicity of biopharmaceuticals. J Biol Chem. 2007;282(4):2229–36.
Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4(6):1332–7.
Caputo N, Jackson MA, Castle JR, El Youssef J, Bakhtiani PA, Bergstrom CP, et al. Biochemical stabilization of glucagon at alkaline pH. Diabetes Technol Ther. 2014;16(11):747–58.
Caputo N, Castle JR, Bergstrom CP, Carroll JM, Bakhtiani PA, Jackson MA, et al. Mechanisms of glucagon degradation at alkaline pH. Peptides. 2013;45:40–7.
Newswanger B, Ammons S, Phadnis N, Ward WK, Castle J, Campbell RW, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol. 2015;9(1):24–33.
Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97(8):2924–35.
Giehm L, Dal Degan F, Klysner S, Fraser PE, Otzen DE. An Aß concatemer with altered aggregation propensities. Biochim Biophys Acta. 2010;1804:2025–35.
Otzen DE. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta. 2017;1859:639–49.
Foley P, Kermanshahi pour A, Beach ES, Zimmerman JB. Derivation and synthesis of renewable surfactants. Chem Soc Rev. 2012;41(4):1499–518.
Respaud R, Marchand D, Parent C, Pelat T, Thullier P, Tournamille JF, et al. Effect of formulation on the stability and aerosol performance of a nebulized antibody. mAbs. 2014;6(5):1347–55.
Otzen DE. Proteins in a brave new surfactant world. Curr Opin Colloid Interface Sci. 2015;20:161–9.
Patil SM, Xu S, Sheftic SR, Alexandrescu AT. Dynamic alpha-helix structure of micelle-bound human amylin. J Biol Chem. 2009;284(18):11982–91.
Sureshbabu N, Kirubagaran R, Jayakumar R. Surfactant-induced conformational transition of amyloid beta-peptide. Eur Biophys J: EBJ. 2009;38(4):355–67.
Giehm L, Oliveira CL, Christiansen G, Pedersen JS, Otzen DE. SDS-induced fibrillation of alpha-synuclein: an alternative fibrillation pathway. J Mol Biol. 2010;401(1):115–33.
Otzen DE, Sehgal P, Westh P. A-lactalbumin is unfolded by all classes of detergents but with different mechanisms. J Colloid Interface Sci. 2009;329(4985):273–83.
Andersen KK, Vad BS, Roelants SL, Van Bogaert IN, Otzen DE. Weak and saturable protein-surfactant interactions in the denaturation of apo-a-lactalbumin by acidic and lactonic sophorolipid. Front Microbiol. 2016;7:1711.
Madsen JK, Pihl R, Moller AH, Madsen AT, Otzen DE, Andersen KK. The anionic biosurfactant rhamnolipid does not denature industrial enzymes. Front Microbiol. 2015;6:292.
Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99(7):2039–44.
Chabenne JR, DiMarchi MA, Gelfanov VM, DiMarchi RD. Optimization of the native glucagon sequence for medicinal purposes. J Diabetes Sci Technol. 2010;4(6):1322–31.
Micsonai A, Wien F, Kernya L, Lee YH, Goto Y, Refregiers M, et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc Natl Acad Sci U S A. 2015;112(24):E3095–103.
Zhang M, Hu R, Liang G, Chang Y, Sun Y, Peng Z, et al. Structural and energetic insight into the cross-seeding amyloid assemblies of human IAPP and rat IAPP. J Phys Chem B. 2014;118(25):7026–36.
Nielsen AD, Arleth L, Westh P. Analysis of protein-surfactant interactions - a titration calorimetric and fluorescence spectroscopic investigation of interactions between Humicola insolens cutinase and an anionic surfactant. Biochim Biophys Acta. 2005;1752(4041):124–32.
Andersen CB, Otzen DE, Christiansen G, Rischel C. Glucagon amyloid-like fibril morphology is selected via morphology-dependent growth inhibition. Biochemistry. 2007;46:7314–24.
Nielsen MM, Andersen KK, Westh P, Otzen DE. Unfolding of beta-sheet proteins in SDS. Biophys J. 2007;92(10):3674–85.
Otzen DE. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta. 2016;1859(4):639–49.
Andersen KK, Otzen DE. Denaturation of alpha-lactalbumin and myoglobin by the anionic biosurfactant rhamnolipid. Biochim Biophys Acta. 2014;1844(12):2338–45.
Jones LS, Bam NB, Randolph TW. Surfactant-stabilized protein formulations: a review of protein-surfactant interactions and novel analytical methodologies. Therapeutic protein and peptide formulation and delivery. ACS Symposium Series. 675: American Chemical Society; 1997. p. 206–22.
Vacha R, Linse S, Lund M. Surface effects on aggregation kinetics of amyloidogenic peptides. J Am Chem Soc. 2014;136(33):11776–82 Epub 2014/07/30. eng.
Zhang D. Polyoxyethylene sorbitan fatty acid esters in Handbook of Pharmaceutical Excipients. In: Rowe RC, Sheskey PJ, Quinn ME, editors. London: Pharmaceutical Press; 2009. p. 549–53.
Marrakchi S, Maibach HI. Sodium lauryl sulfate-induced irritation in the human face: regional and age-related differences. Skin Pharmacol Physiol. 2006;19(3):177–80.
Lee EJ, Rothwell JT. Histological changes to the skin of merino sheep following deep dermal and subcutaneous injections of sodium lauryl sulfate. Aust Vet J. 2010;88(4):146–50.
Hirata Y, Ryu M, Igarashi K, Nagatsuka A, Furuta T, Kanaya S, et al. Natural synergism of acid and lactone type mixed sophorolipids in interfacial activities and cytotoxicities. J Oleo Sci. 2009;58(11):565–72.
Rifkin RA, Maggio ET, Dike S, Kerr DA, Levy M. N-dodecyl-beta-D-maltoside inhibits aggregation of human interferon-beta-1b and reduces its immunogenicity. J NeuroImmune Pharmacol. 2011;6(1):158–62.
Vllasaliu D, Shubber S, Fowler R, Garnett M, Alexander C, Stolnik S. Epithelial toxicity of alkylglycoside surfactants. J Pharm Sci. 2013;102(1):114–25.
Stipcevic T, Piljac A, Piljac G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns. 2006;32(1):24–34.
Jiang L, Shen C, Long X, Zhang G, Meng Q. Rhamnolipids elicit the same cytotoxic sensitivity between cancer cell and normal cell by reducing surface tension of culture medium. Appl Microbiol Biotechnol. 2014;98(24):10187–96.
Hirata Y, Ryu M, Oda Y, Igarashi K, Nagatsuka A, Furuta T, et al. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J Biosci Bioeng. 2009;108(2):142–6.
Zhang Y, Liu C, Dong B, Ma X, Hou L, Cao X, et al. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation. 2015;38(2):756–64.
Acknowledgments and Disclosures
J.K.M. is funded by an Industrial PhD fellowship cofunded by Zealand Pharma A/S and the Danish Innovation Foundation (grant no. 1355–00136).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Madsen, J.K., Giehm, L. & Otzen, D.E. The Use of Surfactants to Solubilise a Glucagon Analogue. Pharm Res 35, 235 (2018). https://doi.org/10.1007/s11095-018-2494-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2494-2