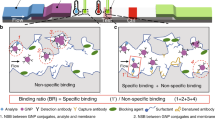
Abstract
Purpose
In order to improve the detection limit of existing HIV diagnostic assays, we explored the use of a temperature-responsive magnetic nanoparticle reagent system in conjunction with cyanovirin-N for HIV recognition to rapidly and efficiently concentrate viral particles from larger sample volumes, ~ 1 ml.
Methods
Cyanovirin-N (CVN) mutant, Q62C, was expressed, biotinylated, and then complexed with a thermally responsive polymer-streptavidin conjugate. Confirmation of protein expression/activity was performed using matrix assisted laser desorption/ionization (MALDI) and a TZM-bl HIV inhibition assay. Biotinylated CVN mutant recognition with gp120 was characterized using surface plasmon resonance (SPR). Virus separation and enrichment using a thermoresponsive magnetic nanoparticle reagent system were measured using RT-PCR.
Results
Biotinylated Q62C exhibited a KD of 0.6 nM to gp120. The temperature-responsive binary reagent system achieved a maximum viral capture of nearly 100% HIV, 1 × 105 virus copies in 100 μl, using pNIPAAm-Q62C within 30 minutes. Additionally, the same reagent system achieved nearly 9-fold enrichment by processing a 10-times larger sample of 1000 μl (Fig. 3).
Conclusion
This work demonstrated a temperature-responsive reagent system that provides enrichment of HIV using antiviral lectin CVN for recognition, which is potentially amenable for use in point-of-care settings.




Similar content being viewed by others
Abbreviations
- CVN:
-
Cyanovirin-N
- HIV:
-
Human immunodeficiency virus
- LCST:
-
Lower critical solution temperature
- LFA:
-
Lateral flow assay
- MALDI:
-
Matrix-assisted laser desorption ionization
- mNP:
-
Magnetic nanoparticles
- pNIPAAm:
-
Poly(N-isopropylacrylamide)
- SA:
-
Streptavidin
- SPR:
-
Surface plasmon resonance
References
da Motta LR, Vanni AC, Kato SK, Borges LGD, Sperhacke RD, Ribeiro RMM, et al. Evaluation of five simple rapid HIV assays for potential use in the Brazilian national HIV testing algorithm. J Virol Methods. 2013;194(1-2):132–7.
Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, et al. Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a national household survey in Swaziland. J Clin Microbiol. 2014;52(10):3743–8.
Fox J, Dunn H, O’Shea S. Low rates of p24 antigen detection using a fourth-generation point of care HIV test. Sex Transm Infect. 2011;87(2):178–9.
Chiu RYT, Jue E, Yip AT, Berg AR, Wang SJ, Kivnick AR, et al. Simultaneous concentration and detection of biomarkers on paper. Lab Chip. 2014;14(16):3021–8.
Jue E, Yamanishi CD, Chiu RYT, Wu BM, Kamei DT. Using an aqueous two-phase polymer-salt system to rapidly concentrate viruses for improving the detection limit of the lateral-flow immunoassay. Biotechnol Bioeng. 2014;111(12):2499–507.
Mashayekhi F, Chiu RYT, Le AM, Chao FC, Wu BM, Kamei DT. Enhancing the lateral-flow immunoassay for viral detection using an aqueous two-phase micellar system. Anal Bioanal Chem. 2010;398(7-8):2955–61.
Corchero JL, Villaverde A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009;27(8):468–76.
Akbarzadeh A, Samiei M, Davaran S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 2012;7(1):144.
Lai JJ, Hoffman JM, Ebara M, Hoffman AS, Estournes C, Wattiaux A, et al. Dual magnetic-/temperature-responsive nanoparticles for microfluidic separations and assays. Langmuir. 2007;23(13):7385–91.
Lai JJ, Nelson KE, Nash MA, Hoffman AS, Yager P, Stayton PS. Dynamic bioprocessing and microfluidic transport control with smart magnetic nanoparticles in laminar-flow devices. Lab Chip. 2009;9(14):1997–2002.
Schild HG. Poly (N-Isopropylacrylamide) - experiment, theory and application. Theory Appl Prog Polym Sci. 1992;17(2):163–249.
Kubota K, Fujishige S, Ando I. Single-chain transition of Poly(N-Isopropylacrylamide) in water. J Phys Chem-Us. 1990;94(12):5154–8.
Heskins M, Guillet JE. Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem. 1968;2(8):1441–55.
Nash MA, Waitumbi JN, Hoffman AS, Yager P, Stayton PS. Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system. ACS Nano. 2012;6(8):6776–85.
Nash MA, Yager P, Hoffman AS, Stayton PS. Mixed stimuli-responsive magnetic and gold nanoparticle system for rapid purification, enrichment, and detection of biomarkers. Bioconjug Chem. 2010;21(12):2197–204.
Stayton PS, Lai J, Nehilla BJ, Srinivasan S, inventors; University of Washington, assignee. Stimuli-responsive polymer diagnostic assay comprising magnetic nanoparticles and capture conjugates. US Patent 9080933. 2015.
Bewley CA, Otero-Quintero S. The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to man(8) D1D3 and Man(9) with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J Am Chem Soc. 2001;123(17):3892–902.
Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, OKeefe BR, Mori T, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Ch. 1997;41(7):1521–30.
O’Keefe BR, Shenoy SR, Xie D, Zhang WT, Muschik JM, Currens MJ, et al. Analysis of the interaction between the HIV-Inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol Pharmacol. 2000;58(5):982–92.
Xiong S, Fan J, Kitazato K. The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biot. 2010;86(3):805–12.
Colleluori DM, Tien D, Kang F, Pagliei T, Kuss R, McCormick T, et al. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr Purif. 2005;39(2):229–36.
Gao XL, Chen W, Guo CW, Qian CW, Liu G, Ge F, et al. Soluble cytoplasmic expression, rapid purification, and characterization of cyanovirin-N as a His-SUMO fusion. Appl Microbiol Biot. 2010;85(4):1051–60.
Mori T, Gustafson KR, Pannell LK, Shoemaker RH, Wu L, McMahon JB, et al. Recombinant production of cyanovirin-N, a potent human immunodeficiency virus-inactivating protein derived from a cultured cyanobacterium. Protein Expr Purif. 1998;12(2):151–8.
Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J Control Release. 2009;133(3):221–9.
Gustafson KR, Sowder 2nd RC, Henderson LE, Cardellina 2nd JH, McMahon JB, Rajamani U, et al. Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (human immunodeficiency virus) protein from the cyanobacterium Nostoc ellipsosporum. Biochem Biophys Res Commun. 1997;238(1):223–8.
Butt TR, Edavettal SC, Hall JP, Mattern MR. SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif. 2005;43(1):1–9.
Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5(1-2):75–86.
Kada G, Kaiser K, Falk H, Gruber HJ. Rapid estimation of avidin and streptavidin by fluorescence quenching or fluorescence polarization. Biochim Biophys Acta. 1999;1427(1):44–8.
WalkerPeach CR, Winkler M, DuBois DB, Pasloske BL. Ribonuclease-resistant RNA controls (Armored RNA) for reverse transcription-PCR, branched DNA, and genotyping assays for hepatitis C virus. Clin Chem. 1999;45(12):2079–85.
Bewley CA, Gustafson KR, Boyd MR, Covell DG, Bax A, Clore GM, et al. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat Struct Biol. 1998;5(7):571–8.
Zappe H, Snell ME, Bossard MJ. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv Drug Deliv Rev. 2008;60(1):79–87.
Tajima N, Takai M, Ishihara K. Significance of antibody orientation unraveled: well-oriented antibodies recorded high binding affinity. Anal Chem. 2011;83(6):1969–76.
Chalmers JJ, Xiong Y, Jin X, Shao M, Tong X, Farag S, et al. Quantification of non-specific binding of magnetic micro- and nanoparticles using cell tracking velocimetry: implication for magnetic cell separation and detection. Biotechnol Bioeng. 2010;105(6):1078–93.
Cheng X, Canavan HE, Graham DJ, Castner DG, Ratner BD. Temperature dependent activity and structure of adsorbed proteins on plasma polymerized N-isopropyl acrylamide. Biointerphases. 2006;1(1):61.
Hoffman JM, Stayton PS, Hoffman AS, Lai JJ. Stimuli-responsive reagent system for enabling microfluidic immunoassays with biomarker purification and enrichment. Bioconjug Chem. 2015;26(1):29–38.
Wu J, Gao L, Gao D. Multistage magnetic separation of microspheres enabled by temperature-responsive polymers. ACS Appl Mater Interfaces. 2012;4(6):3041–6.
Pennypacker C, Perelson AS, Nys N, Nelson G, Sessler DI. Localized or systemic in vivo heat inactivation of human immunodeficiency virus (HIV): a mathematical analysis. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(4):321–9.
Lupo LD, Butera ST. Application of μMACS(TM) Streptavidin MicroBeads for the analysis of HIV-1 directly from patient plasma. MACS More. 2004;8(1):15–9.
Chen GD, Alberts CJ, Rodriguez W, Toner M. Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles. Anal Chem. 2010;82(2):723–8.
Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77(19):10557–65.
Dacheux L, Moreau A, Ataman-Onal Y, Biron F, Verrier B, Barin F. Evolutionary dynamics of the glycan shield of the human immunodeficiency virus envelope during natural infection and implications for exposure of the 2G12 epitope. J Virol. 2004;78(22):12625–37.
Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12.
ACKNOWLEDGMENTS AND DISCLOSURES
We thank Reggie Gausman and Jose Ortega for technical assistance with the RT-PCR work. Funding support was provided by the University of Washington, the University of Washington CFAR Clinical Research and Retrovirology Core (P30-AI-027757) and the ACTG Laboratory Center (UM1-AI-106701), NIH GM100558 and CA174581, and the National Science Foundation Graduate Research Fellowship Program. BJN is an employee of Nexgenia, a company that is commercializing stimuli-responsive reagents for life science applications.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 377 kb)
Rights and permissions
About this article
Cite this article
Phan, J.C., Nehilla, B.J., Srinivasan, S. et al. Human Immunodeficiency Virus (HIV) Separation and Enrichment via the Combination of Antiviral Lectin Recognition and a Thermoresponsive Reagent System. Pharm Res 33, 2411–2420 (2016). https://doi.org/10.1007/s11095-016-1980-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1980-7