ABSTRACT
Purpose
The further characterization of the cell line RPMI 2650 and the evaluation of different culture conditions for an in vitro model for nasal mucosa.
Methods
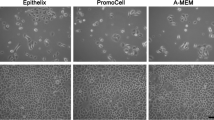
Cells were cultured in media MEM or A-MEM at air-liquid (A-L) or liquid-liquid (L-L) interfaces for 1 or 3 weeks. Different cryopreservation methods and cell culture techniques were evaluated with immunolabelling of junctional proteins, ultrastructural analysis using electron microscopy, transepithelial electrical resistance (TEER) measurements, permeation studies with dextran and jacalin, and gene expression profiling of 84 drug transporters.
Results
Cell proliferation and differentiation depended on the used medium. The established epithelia expressed occludin, claudin-1, and E-cadherin under all conditions. Cells grown at the A-L interface formed more layers and exhibited a higher TEER and lower dextran and jacalin permeability than at the L-L interface, where cells morphologically exhibited a more differentiated phenotype. The expression of ABC and SLC transporters depended on culture duration and interface.
Conclusions
The RPMI 2650 cells form a polarized epithelium resembling nasal mucosa. However, different culture conditions have a significant effect on cell ultrastructure, barrier integrity, and gene expression, and should be considered when using this cell line as an in vitro model for drug permeability studies and screening of nasal drug candidates.










Similar content being viewed by others
Abbreviations
- A :
-
Surface area
- ABC:
-
ATP-binding cassette
- A-L:
-
Air-liquid interface
- c 0 :
-
Initial donor concentration
- dQ/dt :
-
Flux of dextran-FITC or jacalin-fluorescein across the RPMI 2650 cell barrier
- L-L:
-
Liquid-liquid interface
- Papp :
-
Apparent permeability coefficient
- SEM:
-
Scanning electron microscopy
- SLC:
-
Solute carrier
- TEER:
-
Transepithelial electrical resistance
- TEM:
-
Transmission electron microscopy
- TJ:
-
Tight junction
REFERENCES
Ehrhardt C, Laue M, Kim K-J. In Vitro Models of the Alveolar Epithelial Barrier. In: Ehrhardt C, Kim K-J, editors. Drug Absorption Studies. Biotechnology: Pharmaceutical Aspects. VII: Springer US; 2008. p. 258–82.
Audus KL, Bartel RL, Hidalgo IJ, Borchardt RT. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm Res. 1990;7(5):435–51.
Wengst A, Reichl S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2010;74(2):290–7.
Kamekura R, Kojima T, Koizumi J, Ogasawara N, Kurose M, Go M, et al. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338(2):283–93.
Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206.
Merkle HP, Ditzinger G, Lang SR, Peter H, Schmidt MC. In vitro cell models to study nasal mucosal permeability and metabolism. Adv Drug Deliv Rev. 1998;29(1–2):51–79.
De Fraissinette A, Brun R, Felix H, Vonderscher J, Rummelt A. Evaluation of the human cell line RPMI 2650 as an in vitro nasal model. Rhinology. 1995;33(4):194–8.
Werner U, Kissel T. In-vitro cell culture models of the nasal epithelium: a comparative histochemical investigation of their suitability for drug transport studies. Pharm Res. 1996;13(7):978–88.
Bai S, Yang T, Abbruscato TJ, Ahsan F. Evaluation of human nasal RPMI 2650 cells grown at an air-liquid interface as a model for nasal drug transport studies. J Pharm Sci. 2008;97(3):1165–78.
Moore GE, Sandberg AA. Studies of a human tumor cell line with a diploid karyotype. Cancer. 1964;17:170–5.
Moorhead PS. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650). Exp Cell Res. 1965;39(1):190–6.
Kurti L, Veszelka S, Bocsik A, Ozsvari B, Puskas LG, Kittel A, et al. Retinoic acid and hydrocortisone strengthen the barrier function of human RPMI 2650 cells, a model for nasal epithelial permeability. Cytotechnology. 2013;65(3):395–406.
Peter HG. Cell culture sheets to study nasal peptide metabolism: The human nasal RPMI 2650 cell line model. ETH Zurich: Switzerland; 1996.
Salib RJ, Lau LC, Howarth PH. The novel use of the human nasal epithelial cell line RPMI 2650 as an in vitro model to study the influence of allergens and cytokines on transforming growth factor-beta gene expression and protein release. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2005;35(6):811–9.
Ding GQ, Zheng CQ, Liu Y, Tian J. Roles of epidermal growth factor receptor signaling pathway on cultured human nasal epithelial cells RPMI-2650. Zhonghua er bi yan hou tou jing wai ke za zhi. Chin J Otorhinolaryngol Head Neck Surg. 2009;44(3):203–8.
Reichl S, Becker K. Cultivation of RPMI 2650 cells as an in-vitro model for human transmucosal nasal drug absorption studies: optimization of selected culture conditions. J Pharm Pharmacol. 2012;64(11):1621–30.
Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993;268(36):27286–90.
Keenan J, Liang Y, Clynes M. Two-deoxyglucose as an anti-metabolite in human carcinoma cell line RPMI-2650 and drug-resistant variants. Anticancer Res. 2004;24(2A):433–40.
Wilkinson LJ, Duffield ML, Titball RW, Lindsay CD. Down-regulation of gene transcripts associated with ricin tolerance in human RPMI 2650 cells. Toxicol in vitro: Int J Publ Assoc BIBRA. 2007;21(3):509–20.
Visnjar T, Kreft ME. Air-liquid and liquid-liquid interfaces influence the formation of the urothelial permeability barrier in vitro. In Vitro Cell Dev Biol Anim. 2013;49(3):196–204.
Campbell ML, Abboud EC, Dolberg ME, Sanchez JE, Marcet JE, Rasheid SH. Treatment of refractory perianal fistulas with ligation of the intersphincteric fistula tract: preliminary results. Am Surg. 2013;79(7):723–7.
Schmidt MC. Therapeutic peptides: How Do they Get through the nasal epithelium? ETH Zurich: Switzerland; 1999.
Hosoya K, Kubo H, Natsume H, Sugibayashi K, Morimoto Y, Yamashita S. The structural barrier of absorptive mucosae: site difference of the permeability of fluorescein isothiocyanate-labelled dextran in rabbits. Biopharm Drug Dispos. 1993;14(8):685–95.
Takano K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, et al. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem: Off J Histochem Soc. 2005;53(5):611–9.
Kubo H, Hosoya K-I, Natsume H, Sugibayashi K, Morimoto Y. In vitro permeation of several model drugs across rabbit nasal mucosa. Int J Pharm. 1994;103(1):27–36.
Loch C, Zakelj S, Kristl A, Nagel S, Guthoff R, Weitschies W, et al. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2012;47(1):131–8.
Kreft ME, Hudoklin S, Jezernik K, Romih R. Formation and maintenance of blood-urine barrier in urothelium. Protoplasma. 2010;246(1–4):3–14.
Bosquillon C. Drug transporters in the lung–do they play a role in the biopharmaceutics of inhaled drugs? J Pharm Sci. 2010;99(5):2240–55.
Reichl S, Dolberg A. Expression analysis of drug transporter proteins in RPMI 2650 cell line and excised human nasal mucosa. AAPS 2013 Annual Meeting & Exposition; November 10–14 2013; Henry B. Gonzales Convention Center, San Antonio, TX, USA 2013.
Wioland MA, Fleury-Feith J, Corlieu P, Commo F, Monceaux G, Lacau-St-Guily J, et al. CFTR, MDR1, and MRP1 immunolocalization in normal human nasal respiratory mucosa. J Histochem Cytochem: Off J Histochem Soc. 2000;48(9):1215–22.
Genter MB, Krishan M, Augustine LM, Cherrington NJ. Drug transporter expression and localization in rat nasal respiratory and olfactory mucosa and olfactory bulb. Drug Metab Dispos: Biol fate Chem. 2010;38(10):1644–7.
Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I. Intracellular ion channels and cancer. Front Physiol. 2013;4:227.
Agu R, MacDonald C, Cowley E, Shao D, Renton K, Clarke DB, et al. Differential expression of organic cation transporters in normal and polyps human nasal epithelium: implications for in vitro drug delivery studies. Int J Pharm. 2011;406(1–2):49–54.
Karlsson T, Bolshakova A, Magalhaes MA, Loitto VM, Magnusson KE. Fluxes of water through aquaporin 9 weaken membrane-cytoskeleton anchorage and promote formation of membrane protrusions. PLoS ONE. 2013;8(4):e59901.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Sanja Čabraja, Sabina Železnik, Nada Pavlica and Linda Štrus for technical assistance. The study was supported by Lek Pharmaceuticals, d.d., Sandoz Development Center Slovenia and by Slovenian Research Agency (Grants No P3-0108, P1-170).
Author information
Authors and Affiliations
Corresponding author
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
Supplementary Table I
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Kreft, M.E., Jerman, U.D., Lasič, E. et al. The Characterization of the Human Nasal Epithelial Cell Line RPMI 2650 Under Different Culture Conditions and Their Optimization for an Appropriate in vitro Nasal Model. Pharm Res 32, 665–679 (2015). https://doi.org/10.1007/s11095-014-1494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1494-0