ABSTRACT
Purpose
To investigate the in vitro release and degradation of desmopressin from saturated triglyceride microparticles under both lipolytic and proteolytic conditions.
Methods
The release of desmopressin from different solid lipid microparticles in the absence and presence of a microbial lipase and protease was determined. Trilaurin (TG12), trimyristin (TG14), tripalmitin (TG16), and tristearin (TG18) were used as lipid excipients to produce solid lipid microparticles.
Results
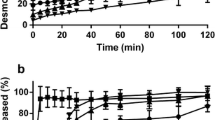
In the presence of lipase, the rate of drug release from different lipid particles was in the order of TG14 > TG16 > TG18, which is the same rank order as the lipid degradation rate. A reverse rank order was found for the protection of desmopressin from enzymatic degradation due to spatial separation of desmopressin from the protease. TG12 accelerated the release of desmopressin from all lipid particles when added as either drug-free microparticles to the lipolysis medium or incorporated in TG16 particles. Additionally, TG12 particles protected desmopressin from degradation when present in the lipolysis medium with the other lipid microparticles.
Conclusions
TG12 is a very interesting lipid for oral lipid formulations containing peptides and proteins as it alters release and degradation of the incorporated desmopressin. The present study demonstrates the possibility of bio-relevant in vitro evaluation of lipid-based solid particles.






Similar content being viewed by others
REFERENCES
Zhang N, Ping Q, Huang G, Xu W, Cheng Y, Han X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327(1–2):153–9.
Lowe PJ, Temple CS. Calcitonin and insulin in isobutylcyanoacrylate nanocapsules: protection against proteases and effect on intestinal absorption in rats. J Pharm Pharmacol. 1994;46(7):547–52.
Damgé C, Michel C, Aprahamian M, Couvreur P, Devissaguet JP. Nanocapsules as carriers for oral peptide delivery. J Control Release. 1990;13(2–3):233–9.
Muranishi S. Modification of intestinal absorption of drugs by lipoidal adjuvants. Pharm Res. 1985;2(3):108–18.
Charman WN, Porter CJH, Mithani S, Dressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86(3):269–82.
Garcia-Fuentes M, Prego C, Torres D, Alonso MJ. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005;25(1):133–43.
Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2(4):743–9.
Salmaso S, Bersani S, Elvassore N, Bertucco A, Caliceti P. Biopharmaceutical characterisation of insulin and recombinant human growth hormone loaded lipid submicron particles produced by supercritical gas micro-atomisation. Int J Pharm. 2009;379(1):51–8.
Christophersen PC, Zhang L, Yang M, Nielsen HM, Müllertz A, Mu H. Solid lipid particles for oral delivery of peptide and protein drugs I - Elucidating the release mechanism of lysozyme during lipolysis. Eur J Pharm Biopharm. 2013;85(3):473–80.
Langguth P, Bohner V, Heizmann J, Merkle HP, Wolffram S, Amidon GL, et al. The challenge of proteolytic enzymes in intestinal peptide delivery. J Control Release. 1997;46(1–2):39–57.
Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev. 1989;4(2):171–207.
Matsui K, Kimura T, Ota K, Iitake K, Shoji M, Inoue M, et al. Resistance of 1-Deamino [8-D-Argininei]-Vasopressin to in vitro degradation as compared with arginine vasopressin. Endocrinol Jpn. 1985;32(4):547–57.
Fredholt K, Østergaard J, Savolainen J, Friis GJ. a-Chymotrypsin-catalyzed degradation of desmopressin (dDAVP): influence of pH, concentration and various cyclodextrins. Int J Pharm. 1999;178(2):223–9.
Reithmeier H, Herrmann J, Göpferich A. Lipid microparticles as a parenteral controlled release device for peptides. J Control Release. 2001;73(2–3):339–50.
Larsen AT, Sassene P, Müllertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int J Pharm. 2011;417(1–2):245–55.
Williams HD, Sassene P, Kleberg K, Bakala-N’Goma JC, Calderone M, Jannin V, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharm Sci. 2012;101(9):3360–80.
Aloulou A, Puccinelli D, de Caro A, Leblond Y, Carrière F. A comparative study on two fungal lipases from Thermomyces lanuginosus and Yarrowia lipolytica shows the combined effects of detergents and pH on lipase adsorption and activity. Biochim Biophys Acta (BBA) - Mol Cell Biol Lipids. 2007;1771(12):1446–56.
Srividhya J, Schnell S. Why substrate depletion has apparent first-order kinetics in enzymatic digestion. Comput Biol Chem. 2006;30(3):209–14.
Schwab M, McGoverin CM, Gordon KC, Winter G, Rades T, Myschik J, et al. Studies on the lipase-induced degradation of lipid-based drug delivery systems. Part II - Investigations on the mechanisms leading to collapse of the lipid structure. Eur J Pharm Biopharm. 2013;84(3):456–63.
Kellens M, Meeussen W, Gehrke R, Reynaers H. Synchrotron radiation investigations of the polymorphic transitions of saturated monoacid triglycerides. Part 1: tripalmitin and tristearin. Chem Phys Lipids. 1991;58(1GÇô2):131–44.
Kellens M, Meeussen W, Hammersley A, Reynaers H. Synchrotron radiation investigations of the polymorphic transitions in saturated monoacid triglycerides. Part 2: polymorphism study of a 50:50 mixture of tripalmitin and tristearin during crystallization and melting. Chem Phys Lipids. 1991;58(1–2):145–58.
Lundin PDP, Bojrup M, Ljusberg-Wahren H, Weström BR, Lundin S. Enhancing effects of monohexanoin and two other medium-chain glyceride vehicles on intestinal absorption of desmopressin (dDAVP). J Pharm Exp Ther. 1997;282(2):585–90.
Mu H, Holm R, Müllertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453(1):215–24.
Small DM. A classification of biologic lipids based upon their interaction in aqueous systems. J Am Oil Chem Soc. 1968;45(3):108–19.
Koynova R, Tenchov B. Interactions of surfactants and fatty acids with lipids. Curr Opin Colloid Interface Sci. 2001;6(3):277–86.
Tilley AJ, Dong YD, Chong JYT, Hanley T, Kirby N, Drummond CJ, et al. Transfer of lipid between triglyceride dispersions and lyotropic liquid crystal nanostructured particles using time-resolved SAXS. Soft Matter. 2012;8(20):5696–708.
Aungst BJ. Intestinal permeation enhancers. J Pharm Sci. 2000;89(4):429–42.
Lindmark T, Nikkilä T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 1995;275(2):958–64.
Jantratid E, Janssen N, Reppas C, Dressman J. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76.
ACKNOWLEDGMENTS AND DISCLOSURES
Dorthe Ørbæk (University of Copenhagen, Denmark) is acknowledged for her technical assistance with SEM imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christophersen, P.C., Zhang, L., Müllertz, A. et al. Solid Lipid Particles for Oral Delivery of Peptide and Protein Drugs II – The Digestion of Trilaurin Protects Desmopressin from Proteolytic Degradation. Pharm Res 31, 2420–2428 (2014). https://doi.org/10.1007/s11095-014-1337-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1337-z