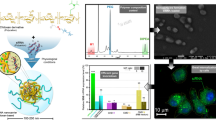
Abstract
Purpose
Development of RNA interference based therapeutics for neurological and neurodegenerative diseases is hindered by a lack of non-viral vectors with suitable properties for systemic administration. Amphiphilic and cationic cyclodextrins (CD) offer potential for neuronal siRNA delivery. We aimed to improve our CD-based siRNA formulation through incorporation of a polyethyleneglycol (PEG) shielding layer and a cell penetrating peptide, octaarginine (R8).
Methods
CD.siRNA complexes were modified by addition of an R8-PEG-lipid conjugate. Physical properties including size, charge and stability were assessed. Flow cytometry was used to determine uptake levels in a neuronal cell model. Knockdown of an exogenous gene and an endogenous housekeeping gene were used to assess gene silencing abilities.
Results
CD.siRNA complexes modified with R8-PEG-lipid exhibited a lower surface charge and greater stability to a salt-containing environment. Neuronal uptake was increased and significant reductions in the levels of two target genes were achieved with the new formulation. However, the PEG layer was not sufficient to protect against serum-induced aggregation.
Conclusions
The R8-PEG-lipid-CD.siRNA formulation displayed enhanced salt-stability due to the PEG component, while the R8 component facilitated transfection of neuronal cells and efficient gene silencing. Further improvements will be investigated in the future in order to optimise stability in serum and enhance neuronal specificity.





Similar content being viewed by others
Abbreviations
- CD:
-
cyclodextrin
- CNS:
-
central nervous system
- DIW:
-
deionised water
- DLS:
-
dynamic light scattering
- DMSO:
-
dimethylsulphoxide
- DSPE:
-
1,2-distearoyl-sn-glycero-3-phosphoethanolamine
- GAPDH:
-
glyceraldehyde phosphate dehydrogenase
- MR:
-
mass ratio
- mRNA:
-
messenger RNA
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- ns:
-
non-silencing
- PAMAM:
-
polyamidoamine
- PEG:
-
polyethyleneglycol
- PLL:
-
poly-L-lysine
- R8:
-
octaarginine
- RLU:
-
relative luminescence units
- shRNA:
-
short hairpin RNA
- siRNA:
-
small interfering RNA
References
Maxwell MM. RNAi Applications in Therapy Development for Neurodegenerative Disease. Curr Pharm Des. 2009;15(34):3977–91.
Davidson BL, McCray Jr PB. Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–40.
Thakker DR, Hoyer D, Cryan JF. Interfering with the brain: Use of RNA interference for understanding the pathophysiology of psychiatric and neurological disorders. Pharmacol Ther. 2006;109(3):413–38.
Bergen JM, Park IK, Horner PJ, Pun SH. Nonviral approaches for neuronal delivery of nucleic acids. Pharm Res. 2008;25(5):983–98.
McMahon A, Gomez E, Donohue R, Forde D, Darcy R, O’Driscoll CM. Cyclodextrin gene vectors: Cell trafficking and the influence of lipophilic chain length. J Drug Delivery Sci Technol. 2008;18(5):303–7.
McMahon A, O’Neill MJ, Gomez E, Donohue R, Forde D, darcy R. Targeted gene delivery to hepatocytes with galactosylated amphiphilic cyclodextrins. J Pharm Pharmacol. 2012;64(8):1063–73.
O’ Neill MJ, Guo J, Byrne C, Darcy R, O’ Driscoll CM. Mechanistic studies on the uptake and intracellular trafficking of novel cyclodextrin transfection complexes by intestinal epithelial cells. Int J Pharm. 2011;413(1–2):174–83.
Guo J, Ogier JR, Desgranges S, Darcy R, O’Driscoll C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials. 2012;33(31):7775–84.
Díaz-Moscoso A, Guilloteau N, Bienvenu C, Méndez-Ardoy A, Jiménez Blanco JL, Benito JM, et al. Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery. Biomaterials. 2011;32(29):7263–73.
Diaz-Moscoso A, Le Gourrierec L, Gomez-Garcia M, Benito JM, Balbuena P, Ortega-Caballero F, et al. Polycationic Amphiphilic Cyclodextrins for Gene Delivery: Synthesis and Effect of Structural Modifications on Plasmid DNA Complex Stability, Cytotoxicity, and Gene Expression. Chem—Eur J. 2009;15(46):12871–88.
Mellet CO, Fernandez JMG, Benito JM. Cyclodextrin-based gene delivery systems. Chem Soc Rev. 2011;40(3):1586–608.
Cryan SA, Donohue R, Ravo BJ, Darcy R, O’Driscoll CM. Cationic cyclodextrin amphiphiles as gene delivery vectors. J Drug Delivery Sci Technol. 2004;14(1):57–62.
O’Mahony AM, Doyle D, Darcy R, Cryan JF, O’ Driscoll CM. Characterisation of cationic amphiphilic cyclodextrins for neuronal delivery of siRNA: effect of reversing primary and secondary face modifications. Eur J Pharm Sci. 2012;47:896–903.
Heidel JD, Yu ZP, Liu JYC, Rele SM, Liang YC, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007;104(14):5715–21.
Arima H, Yamashita S, Mori Y, Hayashi Y, Motoyama K, Hattori K, et al. In Vitro and In Vivo gene delivery mediated by Lactosylated Dendrimer/alpha-Cyclodextrin Conjugates (G2) into Hepatocytes. J Controlled Release. 2010;146(1):106–17.
O’Mahony AM, Godinho BMDC, Ogier J, Devocelle M, Darcy R, Cryan JF, et al. Click-Modified Cyclodextrins as Nonviral Vectors for Neuronal siRNA Delivery. ACS Chem Neurosci. 2012;3(10):744–52.
Kostarelos K, Miller AD. Synthetic, self-assembly ABCD nanoparticles; a structural paradigm for viable synthetic non-viral vectors. Chem Soc Rev. 2005;34(11):970–94.
Kim H-K, Davaa E, Myung C-S, Park J-S. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int J Pharm. 2010;392(1–2):141–7.
Guo J, Fisher KA, Darcy R, Cryan JF, O’Driscoll C. Therapeutic targeting in the silent era: advances in non-viral siRNA delivery. Mol Biosyst. 2010;6(7):1143–61.
Guo J, Cheng WP, Gu J, Ding C, Qu X, Yang Z, et al. Systemic delivery of therapeutic small interfering RNA using a pH-triggered amphiphilic poly-l-lysine nanocarrier to suppress prostate cancer growth in mice. Eur J Pharm Sci. 2012;45:521–32.
O’Mahony AM, Ogier J, Desgranges S, Cryan JF, Darcy R, O’ Driscoll CM. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: co-formulation opportunities for siRNA delivery. Org Biomol Chem. 2012;10(25):4954–60.
Schäfer J, Höbel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010;31(26):6892–900.
Vader P, van der Aa LJ, Engbersen JFJ, Storm G, Schiffelers RM. Physicochemical and Biological Evaluation of siRNA Polyplexes Based on PEGylated Poly(amido amine)s. Pharm Res. 2012;29(2):352–61.
Nakamura Y, Kogure K, Futaki S, Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J Controlled Release. 2007;119(3):360–7.
Khalil IA, Kogure K, Futaki S, Hama S, Akita H, Ueno M, et al. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene Ther. 2007;14(8):682–9.
Kim SW, Kim NY, Choi YB, Park SH, Yang JM, Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J Controlled Release. 2010;143(3):335–43.
Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim Y-H, et al. Cholesteryl Oligoarginine Delivering Vascular Endothelial Growth Factor siRNA Effectively Inhibits Tumor Growth in Colon Adenocarcinoma. Mol Ther. 2006;14(3):343–50.
Tonges L, Lingor P, Egle R, Dietz GPH, Fahr A, Bahr M. Stearylated octaarginine and artificial virus-like particles for transfection of siRNA into primary rat neurons. RNA. 2006;12(7):1431–8.
Ifediba MA, Medarova Z, Ng SW, Yang JZ, Moore A. siRNA Delivery to CNS Cells using a Membrane Translocation Peptide. Bioconjugate Chem. 2010;21(5):803–6.
Kim ID, Lim CM, Kim JB, Nam HY, Nam K, Kim SW, et al. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Controlled Release. 2010;142(3):422–30.
Kim J-B, Choi JS, Nam K, Lee M, Park J-S, Lee J-K. Enhanced transfection of primary cortical cultures using arginine-grafted PAMAM dendrimer, PAMAM-Arg. J Controlled Release. 2006;114(1):110–7.
Perrier T, Saulnier P, Benoît J-P. Methods for the Functionalisation of Nanoparticles: New Insights and Perspectives. Chem—Eur J. 2010;16(38):11516–29.
Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999;460(1):129–33.
Allen TM, Sapra P, Moase E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell Mol Biol Lett. 2002;7(2):217–9.
Béduneau A, Saulnier P, Hindré F, Clavreul A, Leroux J-C, Benoit J-P. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’ fragments. Biomaterials. 2007;28(33):4978–90.
Mendonca LS, Firmino F, Moreira JN, de Lima MCP, Simoes S. Transferrin Receptor-Targeted Liposomes Encapsulating anti-BCR-ABL siRNA or asODN for Chronic Myeloid Leukemia Treatment. Bioconjugate Chem. 2010;21(1):157–68.
Nchinda G, Zschornig O, Uberla K. Increased non-viral gene transfer levels in mice by concentration of cationic lipid DNA complexes formed under optimized conditions. J Gene Med. 2003;5(8):712–22.
Howard KA, Li XW, Somavarapu S, Singh J, Green N, Atuah KN, et al. Formulation of a microparticle carrier for oral polyplex-based DNA vaccines. Biochim Biophys Acta, Gen Subj. 2004;1674(2):149–57.
Tsutsumi T, Hirayama F, Uekama K, Arima H. Potential use of polyamidoamine dendrimer/alpha-cyclodextrin conjugate (generation 3, G3) as a novel carrier for short hairpin RNA-expressing plasmid DNA. J Pharm Sci. 2008;97(8):3022–34.
Liu Y, Reineke TM. Poly(glycoamidoamine)s for Gene Delivery: Stability of Polyplexes and Efficacy with Cardiomyoblast Cells. Bioconjugate Chem. 2005;17(1):101–8.
Srinivasachari S, Liu Y, Prevette LE, Reineke TM. Effects of trehalose click polymer length on pDNA complex stability and delivery efficacy. Biomaterials. 2007;28(18):2885–98.
Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AMF, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinol. 2004;145(1):393–400.
Cardoso ALC, Simoes S, de Almeida LP, Plesnila N, de Lima MCP, Wagner E, et al. Tf-lipoplexes for neuronal siRNA delivery: A promising system to mediate gene silencing in the CNS. J Controlled Release. 2008;132(2):113–23.
Wong YY, Markham K, Xu ZP, Chen M, Lu GQ, Bartlett PF, et al. Efficient delivery of siRNA to cortical neurons using layered double hydroxide nanoparticles. Biomaterials. 2010;31(33):8770–9.
Read ML, Mir S, Spice R, Seabright RJ, Suggate EL, Ahmed Z, et al. Profiling RNA interference (RNAi)-mediated toxicity in neural cultures for effective short interfering RNA design. J Gene Med. 2009;11(6):523–34.
Crombez L, Aldrian-Herrada G, Konate K, Nguyen QN, McMaster GK, Brasseur R, et al. A New Potent Secondary Amphipathic Cell-penetrating Peptide for siRNA Delivery Into Mammalian Cells. Mol Ther. 2009;17(1):95–103.
Inoue Y, Kurihara R, Tsuchida A, Hasegawa M, Nagashima T, Mori T, et al. Efficient delivery of siRNA using dendritic poly(L-lysine) for loss-of-function analysis. J Controlled Release. 2008;126(1):59–66.
Simen BB, Duman CH, Simen AA, Duman RS. TNF alpha signaling in depression and anxiety: Behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59(9):775–85.
Li S-D, Chen Y-C, Hackett MJ, Huang L. Tumor-targeted Delivery of siRNA by Self-assembled Nanoparticles. Mol Ther. 2007;16(1):163–9.
Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97.
Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate Chem. 2007;18(2):456–68.
Xia CF, Boado RJ, Pardridge WM. Antibody-Mediated Targeting of siRNA via the Human Insulin Receptor Using Avidin-Biotin Technology. Mol Pharm. 2009;6(3):747–51.
Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10(11):3667–77.
Ping Y, Liu C, Zhang Z, Liu KL, Chen J, Li J. Chitosan-graft-(PEI-beta-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials. 2011;32(32):8328–41.
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotech. 2005;23(8):1002–7.
Kano A, Moriyama K, Yamano T, Nakamura I, Shimada N, Maruyama A. Grafting of poly(ethylene glycol) to poly-lysine augments its lifetime in blood circulation and accumulation in tumors without loss of the ability to associate with siRNA. J Controlled Release. 2011;149(1):2–7.
de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int J Pharm. 2007;331(2):167–75.
Perez-Martinez FC, Guerra J, Posadas I, Cena V. Barriers to Non-Viral Vector-Mediated Gene Delivery in the Nervous System. Pharm Res. 2011;28(8):1843–58.
Martin-Herranz A, Ahmad A, Evans HM, Ewert K, Schulze U, Safinya CR. Surface functionalized cationic lipid-DNA complexes for gene delivery: PEGylated lamellar complexes exhibit distinct DNA-DNA interaction regimes. Biophys J. 2004;86(2):1160–8.
Zhang C, Tang N, Liu X, Liang W, Xu W, Torchilin VP. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J Controlled Release. 2006;112(2):229–39.
Ho EA, Ramsay E, Ginj M, Anantha M, Bregman I, Sy J, et al. Characterization of cationic liposome formulations designed to exhibit extended plasma residence times and tumor vasculature targeting properties. J Pharm Sci. 2010;99(6):2839–53.
Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13(6):541–52.
Kumar P, Wu HQ, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–U2.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnol. 2011;29(4):341–U179.
Cardoso ALC, Costa P, de Almeida LP, Simoes S, Plesnila N, Culmsee C, et al. Tf-lipoplex-mediated c-Jun silencing improves neuronal survival following excitotoxic damage in vivo. J Controlled Release. 2010;142(3):392–403.
Acknowledgments AND DISCLOSURES
The authors wish to acknowledge Science Foundation Ireland (Strategic Research Cluster grant no. 07/SRC/B1154), the Irish Drug Delivery Network and the Irish Research Council for Science, Engineering and Technology (scholarship to A.O’Mahony) for research funding. We also wish to acknowledge Dr. Matt Gooding for assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Size intensity profiles of (a) MR 20 0% R8 and (b) MR 20 20% R8 after incubation in FBS for 0, 2, 24 and 72 h. (JPEG 44 kb)
Rights and permissions
About this article
Cite this article
O’Mahony, A.M., Desgranges, S., Ogier, J. et al. In Vitro Investigations of the Efficacy of Cyclodextrin-siRNA Complexes Modified with Lipid-PEG-Octaarginine: Towards a Formulation Strategy for Non-viral Neuronal siRNA Delivery. Pharm Res 30, 1086–1098 (2013). https://doi.org/10.1007/s11095-012-0945-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0945-8