ABSTRACT
Purpose
The aim of the present study is to evaluate the formulation effect on the oral absorption of poorly water-soluble drugs using a dissolution/permeation system (D/P system).
Methods
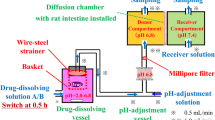
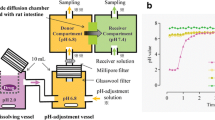
This D/P system, consisting of apical and basal chambers and a Caco-2 cell monolayer mounted between chambers, can be used to perform simultaneous analysis of drug dissolution and permeation process of drugs applied as various dosage forms. Oral administration study with rats was also performed for both drugs as the same dosage forms.
Results
When danazol, a low-soluble and high-permeable drug, was applied to the D/P system as various formulations, dissolved and permeated amounts were significantly high compared with those from a suspension form. On the other hand, whereas the dissolved amount of pranlukast, a low-soluble and low-permeable drug, was significantly increased by formulations, there were no significant changes observed in the permeated amount between suspension and formulation. The oral availability of danazol was significantly increased by formulations but not pranlukast, which corresponded well to in vitro evaluations.
Conclusion
These results indicated that the D/P system might be applicable for selection of formulation on the basis of physicochemical drug properties.





Similar content being viewed by others
REFERENCES
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317(1):61–8.
Hamaguchi T, Shinkuma D, Irie T, Yamanaka Y, Morita Y, Iwamoto B, et al. Effect of a high-fat meal on the bioavailability of phenytoin in a commercial powder with a large particle size. Int J Clin Pharmacol Ther Toxicol. 1993;31(7):326–30.
Kennedy M, Hu J, Gao P, Li L, Ali-Reynolds A, Chal B, et al. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Mol Pharm. 2008;5(6):981–93.
Gao P. Amorphous pharmaceutical solids: characterization, stabilization, and development of marketable formulations of poorly soluble drugs with improved oral absorption. Mol Pharm. 2008;5(6):903–4.
Chokshi RJ, Zia H, Sandhu HK, Shah NH, Malick WA. Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution: pros and cons. Drug Deliv. 2007;14(1):33–45.
Guzmán HR, Tawa M, Zhang Z, Ratanabanangkoon P, Shaw P, Gardner CR, et al. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J Pharm Sci. 2007;96(10):2686–702.
Bak A, Gore A, Yanez E, Stanton M, Tufekcic S, Syed R, et al. The co-crystal approach to improve the exposure of a water-insoluble compound: AMG 517 sorbic acid co-crystal characterization and pharmacokinetics. J Pharm Sci. 2008;97(9):3942–56.
Shiraki K, Takata N, Takano R, Hayashi Y, Terada K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm Res. 2008;25(11):2581–92.
Lu GW, Hawley M, Smith M, Geiger BM, Pfund W. Characterization of a novel polymorphic form of celecoxib. J Pharm Sci. 2006;95(2):305–17.
Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212(2):233–46.
Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
Gao P, Akrami A, Alvarez F, Hu J, Li L, Ma C, et al. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J Pharm Sci. 2009;98(2):516–28.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20(10):1674–80.
Kataoka M, Masaoka Y, Sakuma S, Yamashita S. Effect of food intake on the oral absorption of poorly water-soluble drugs: in vitro assessment of drug dissolution and permeation assay system. J Pharm Sci. 2006;95(9):2051–61.
Kataoka M, Itsubata S, Masaoka Y, Sakuma S, Yamashita S. In vitro dissolution/permeation system to predict the oral absorption of poorly water-soluble drugs: effect of food and dose strength on it. Biol Pharm Bull. 2011;34(3):401–7.
Buch P, Langguth P, Kataoka M, Yamashita S. IVIVC in oral absorption for fenofibrate immediate release tablets using a dissolution/permeation system. J Pharm Sci. 2009;98(6):2001–9.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Khoo SM, Porter CJ, Charman WN. The formulation of Halofantrine as either non-solubilizing PEG 6000 or solubilizing lipid based solid dispersions: physical stability and absolute bioavailability assessment. Int J Pharm. 2000;205(1–2):65–78.
Kim JY, Ku YS. Enhanced absorption of indomethacin after oral or rectal administration of a self-emulsifying system containing indomethacin to rats. Int J Pharm. 2000;194(1):81–9.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, et al. The Solubility-Permeability Interplay: Mechanistic Modeling and Predictive Application of the Impact of Micellar Solubilization on Intestinal Permeation. Mol Pharm. 2011; in press.
Katneni K, Charman SA, Porter CJ. Impact of cremophor-EL and polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. 2007;96(2):280–93.
Takahashi Y, Kondo H, Yasuda T, Watanabe T, Kobayashi S, Yokohama S. Common solubilizers to estimate the Caco-2 transport of poorly water-soluble drugs. Int J Pharm. 2002;246(1–2):85–94.
Hugger ED, Audus KL, Borchardt RT. Effects of poly(ethylene glycol) on efflux transporter activity in Caco-2 cell monolayers. J Pharm Sci. 2002;91(9):1980–90.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10(3):195–204.
Sugano K, Kataoka M, Mathews Cda C, Yamashita S. Prediction of food effect by bile micelles on oral drug absorption considering free fraction in intestinal fluid. Eur J Pharm Sci. 2010;40(2):118–24.
Sunesen VH, Vedelsdal R, Kristensen HG, Christrup L, Müllertz A. Effect of liquid volume and food intake on the absolute bioavailability of danazol, a poorly soluble drug. Eur J Pharm Sci. 2005;24(4):297–303.
Nakajima M, Kanamaru M, Umematsu T, Tsubokura S. A phase I clinical study of a leukotriene C4, D4 and E4 receptor antagonist; ONO-1078 in healthy volunteers. Rynsho iyaku 1993;Suppl. 1:9.
ACKNOWLEDGMENTS & DISCLOSURES
In vitro experiments with the D/P system were financially supported by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, M., Sugano, K., da Costa Mathews, C. et al. Application of Dissolution/Permeation System for Evaluation of Formulation Effect on Oral Absorption of Poorly Water-Soluble Drugs in Drug Development. Pharm Res 29, 1485–1494 (2012). https://doi.org/10.1007/s11095-011-0623-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0623-2