ABSTRACT
Purpose
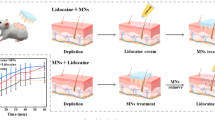
To demonstrate rapid (~1 min) lidocaine delivery using 3M’s solid microstructured transdermal system (sMTS) for prolonged, local analgesic action.
Methods
Polymeric microneedles were fabricated via injection molding and then dip-coated using an aqueous lidocaine formulation. The amount of lidocaine coated onto the microneedles was determined by high performance liquid chromatography (HPLC). To assess drug delivery and dermal pharmacokinetics, lidocaine-coated microneedles were inserted into domestic swine. Skin punch biopsies were collected and analyzed to determine lidocaine concentration in skin using HPLC-mass spectrometry (LC-MS). Commercial lidocaine/prilocaine EMLA (Eutectic Mixture of Local Anesthetic) cream was used as comparative control.
Results
Lidocaine dissolves rapidly off the microneedles and into skin such that the 1-min wear time achieves or exceeds lidocaine tissue levels needed to cause analgesia. This therapeutic threshold (100 ng/mg) was estimated by measuring the total amount of lidocaine and prilocaine in skin following a 1 h EMLA application. When co-formulated with 0.03 wt% vasoconstrictor-epinephrine, the concentration of lidocaine in tissue was maintained above 100 ng/mg for approximately 90 min.
Conclusions
3M’s sMTS can be used to provide rapid delivery of lidocaine for local analgesia up to 90 min.






Similar content being viewed by others
REFERENCES
Guy RH. Current status and future prospects of transdermal drug delivery. Pharm Res. 1996;13:1765–9.
Hallen B, Olsson GL, Uppfeldt A. Pain-free venepuncture. Effect of timing of application of local anaesthetic cream. Anaesthesia. 1984;39:969–72.
Teillol-Foo WLM, Kassab JY. Topical glyceryl trinitrate and eutectic mixture of local anaesthetics in children. A randomised controlled trial on choice of site and ease of venous cannulation. Anaesthesia. 1991;46:881–4.
Milewski M, Brogden NK, Stinchcomb AL. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin Drug Del. 2010;7:617–29.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, et al. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366:190–200.
Peterson TA. Microstructured transdermal systems for intradermal vaccine and drug delivery. Pharm Tech Eur. 2006;18:21–6.
Hansen K, Haldin B. A Solid Microstructured Transdermal System (sMTS) for systemic delivery of salts & proteins. Drug Deliv Tech. 2008;8:38–42.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11.
Kreilgaard M. Dermal pharmacokinetics of microemulsion formulations determined by in vivo microdialysis. Pharm Res. 2001;18:367–73.
Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release. 2000;69:421–33.
Li X, Zhao R, Qin Z, Zhang J, Zhai S, Qiu Y, et al. Microneedle pretreatment improves efficacy of cutaneous topical anesthesia. Am J Emerg Med. 2010;28:130–4.
Singh P, Roberts MS. Dermal and underlying tissue pharmacokinetics of lidocaine after topical application. J Pharm Sci. 1994;83:774–82.
Rosenberg PH. Additives to increase tissue spread of local anesthetics. Tech Reg Anesth Pain Manag. 2004;8:114–8.
Lewis-Smith PA. Adjunctive use of hyaluronidase in local anaesthesia. Br J Plast Surg. 1986;39:554–8.
Moorthy SS, Dierdorf SF, Yaw PB. Influence of volume on the spread of local anesthetic-methylene blue solution after injection for intercostal block. Anesth Analg. 1992;75:389–91.
Singh P, Roberts MS. Effects of vasoconstriction on dermal pharmacokinetics and local tissue distribution of compounds. J Pharm Sci. 1994;83:783–91.
Bernards CM, Kopacz DJ. Effect of epinephrine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology. 1999;91:962–8.
Scott DB, Jebson PJR, Braid DP, Örtengren B, Frisch P. Factors affecting plasma levels of lignocaine and prilocaine. Br J Anaesth. 1972;44:1040–9.
Yamazaki T, Mamiya H, Ichinohe T, Kaneko Y. Distribution of lidocaine in alveolar tissues in rabbits. J Hard Tissue Biol. 2009;18:95–100.
Friedman PM, Mafong EA, Friedman ES, Geronemus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg. 2001;27:1019–26.
Mehra P, Caiazzo A, Maloney P. Lidocaine toxicity. Anesth Prog. 1998;45:38–41.
ACKNOWLEDGMENTS & DISCLOSURES
We would like to thank JoAnn Oesterich, Mary Hopp, Chris Webb and Tonya Grunwald for their assistance with the animal studies. The authors would also like to thank Daniel Duan, Jerry Gysbers, Peter Johnson and David Brandwein for valuable technical discussion. The authors thank Ryan Simmers and Ron Krienke for supplying microneedles.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Brown, K., Siebenaler, K. et al. Development of Lidocaine-Coated Microneedle Product for Rapid, Safe, and Prolonged Local Analgesic Action. Pharm Res 29, 170–177 (2012). https://doi.org/10.1007/s11095-011-0524-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0524-4