ABSTRACT
Purpose
The aim of this work was to investigate the influence of the oily nucleus composition on physico-chemical properties and anesthetic activity of poly (lactide-co-glycolide) nanocapsules with benzocaine.
Methods
Nanocapsules containing benzocaine were prepared with three different oily nucleus composition and characterized by mean diameter, polydispersivity, zeta potential, pH and stability were investigated as a function of time. In vitro release kinetics were performed in a system with two compartments separated by a cellulose membrane. Intensity and duration of analgesia were evaluated in rats by sciatic nerve blockade.
Results
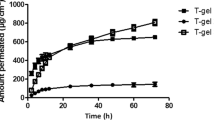
The greatest stability, slower release profile and improvement in the local anesthetic activity of BZC were obtained with the formulation using USP mineral oil as component.
Conclusions
Results from our study provide useful perspectives on selection of the primary materials needed to produce suspensions of polymeric nanocapsules able to act as carriers of BZC, with potential future application in the treatment of pain.









Similar content being viewed by others
REFERENCES
Soppimath KS, Aminabhavi TM, KulkarnI AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20.
Nurkeeva ZS, Mun GA, Khutoryanskiy VV, Bitekenova AB, Dzhusupbekova AB. Polymeric complexes of lidocaine hydrochloride with poly (acrylic acid) and poly (2-hydroxyethyl vinyl ether). J Biomater Sci Polym. 2002;13:759–68.
Schaffazick SR, Guterres SS, Freitas LL, Pohlmann AR. Caracterização e estabilidade fisico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quim Nova. 2003;26:726–37.
Shenoy DB, Amiji MM. Poly (ethylene oxide)-modified poly (ε-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293:261–70.
Mohanraj VJ, Chen Y. Nanoparticles—a review. Trop J Pharm Res. 2006;5:561–73.
Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Control Release. 2008;128:185–99.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–42.
Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: NBM. 2006;2:8–20.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18.
Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiol. 1990;72:711–34.
Fraceto LF, Oyama Jr S, Nakaie CR, Spisni A, de Paula E, Pertinhez TA. Interaction of local anesthetics with a peptide encompassing the IV/S4-S5 linker of the Na+ channel. Biophys Chem. 2006;20:29–39.
de Araújo DR, Tsuneda SS, Cereda CMS, Carvalho FGF, Preté PSC, Fernandes SA, et al. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-beta-cyclodextrin inclusion complex. Eur J Pharm Sci. 2008;33:60–71.
de Paula E, Jarrel HC, Schreier S, Fraceto LF. Preferential location of lidocaine and etidocaine in lecithin bilayers as determined by EPR, fluorescence and 2H-NMR. Biophys Chem. 2008;132:47–54.
de Paula E, Cereda CMS, Tófoli GR, Franz-Montan M, Fraceto LF, de Araújo DR. Drug delivery systems for local anesthetics. Recent Pat Drug Deliv Formul. 2010;4:23–34.
de Jong RH. Local anesthetics. Springfield: C. C. Thomas; 1994.
Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia. Drug Saf. 1996;14:394–405.
So T, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22:335–9.
Ellis BF, Seiler JG, Palmore MM. Methemoglobinemia: a complication after fiberoptic orotracheal intubation with benzocaine spray. A case report. J Bone Joint Surg Am. 1995;77:937–9.
Pinto LMA, Yokaichiya DK, Fraceto LF, de Paula E. Interaction of benzocaine with model membranes. Biophys Chem. 2000;87:213–23.
Kuzma PJ, Kline MD, Calkins MD, Staats PS. Progress in the development of ultra-long-acting local anesthetics. Reg Anesth Pain Med. 1997;22:543–51.
de Araújo DR, Pinto LMA, Braga AFA, de Paula E. Formulações de anestésicos locais de liberação controlada: aplicações terapêuticas. Rev Bras Anestesiol. 2003;53:663–71.
Gorner T, Gref R, Michenot D, Sommer F, Tran MN, Dellacherie E. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J Control Release. 1999;57:259–68.
Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999;57:171–85.
Polakovic M, Gorner T, Gref R, Dellacherie E. Lidocaine loaded biodegradable nanospheres II: Modelling of drug release. J Control Release. 1999;60:169–77.
Govender T, Riley T, Ehtezazi T, Garnett MC, Stolnik S, Illum L, et al. Defining the drug incorporation properties of PLA-PEG nanoparticles. Int J Pharm. 2000;199:95–110.
Dollo G, Le Corre P, Chevanne F, Le Verge R. Inclusion complexation of amide-type local anesthetics with β-cyclodextrin and derivates. II. Evaluation of affinity constants and in vitro transfer rate constants. Int J Pharm. 1996;136:165–74.
Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. Pharm Sci. 1997;86:147–62.
Loftsson T, Másson M. Cyclodextrins in topical drug formulations: theory and practice. Int J Pharm. 2001;225:15–30.
Oliveira AG, Scarpa MV, Correa MA, Cera LFR, Formariz TP. Microemulsões: estrutura e aplicações como sistema de liberação de fármacos. Quim Nova. 2004;27:131–8.
Pinto LMA, Fraceto LF, Santana MHA, Pertinhez TA, Junior SO, de Paula E. Physicochemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J Pharm Biomed Anal. 2005;39:956–63.
de Araújo DR, Braga AFA, Moraes CM, Fraceto LF, de Paula E. Mistura com excesso enantiomérico de 50% (S75-R25) de bupivacaína complexada com ciclodextrinas e anestesia por via subaracnóidea em ratos. Rev Bras Anestesiol. 2006;56:495–506.
Moraes CM, Abrami P, de Araújo DR, Braga AFA, Issa MG, Ferraz HG, et al. Characterization of lidocaine:hydroxypropyl-β-cyclodextrin inclusion complex. J Incl Phenom Macrocycl Chem. 2007;57:313–6.
Moraes CM, Abrami P, de Paula E, Braga AFA, Fraceto LF. HPLC and solubility study of interaction between S (−) bupivacaine and hydroxypropyl-β-cyclodextrin. Int J Pharm. 2007;331:99–106.
Moraes CM, Abrami P, de Paula E, Andreo-Filho N, Fraceto LF. Preparo e caracterização físico-química de complexos de inclusão entre anestésicos locais e hidroxipropil-β-ciclodextrina. Quim Nova. 2007;30:777–84.
Le Guévello P, Le Corre P, Chevanne F, Le Verge R. High-performance liquid chromatographic determination of bupivacaine in plasma samples for biopharmaceutical studies and applications to seven other local anesthetics. J Chromatogr. 1993;622:284–90.
Grant GJ, Bansinath M. Liposomal delivery systems for local anesthetics. Reg Anesth Pain Med. 2001;26:61–3.
Grant SA. The Holy Grail: long-acting local anesthetics and liposomes. Best Pract Res Clin Anaesth. 2002;16:345–52.
Fraceto LF, Pinto LMA, Franzoni L, Braga AC, Spisni A, Schreier S, et al. Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophys Chemist. 2002;99:229–43.
de Araújo DR, Cereda CMS, Brunetto GB, Pinto LMA, Santana MHA, de Paula E. Encapsulation of mepivacaine prolongs the analgesia provided by sciatic nerve blockade in mice. Can J Anaesth. 2004;51:566–72.
de Araújo DR, Cereda CMS, Brunetto GB, Vomero VU, Pierucci A, Santo Neto H, et al. Pharmacological and local toxicity studies of a liposomal formulation for the novel local. J Pharm Pharmacol. 2008b;60:1449–57.
Cereda CMS, de Araújo DR, Brunetto GB, de Paula E. Liposomal prilocaine: preparation, characterization and in vivo evaluation. J Pharm Pharmacol Sci. 2004;7:235–40.
Colombo G, Padera R, Langer R, Kohane DS. Prolonged duration anesthesia with lipid-protein-sugar particles containing bupivacaine and dexamethasone. J Biomed Mater Res A. 2005;75:458–64.
Rose JS, Neal JM, Kopacz DJ. Extended-duration analgesia: update on microspheres and liposomes. Reg Anesth Pain Med. 2005;30:275–85.
Moraes CM, de Matos AP, de Paula E, Rosa AH, Fraceto LF. Benzocaine loaded biodegradable poly-(d, l-lactide-co-glycolide) nanocapsules: factorial design and characterization. Mater Sci Eng B. 2009;165:243–6.
Grillo R, Melo NFS, de Araújo DR, de Paula E, Dias Filho NL, Rosa AH, et al. Validation of an HPLC method for quantitative determination of benzocaine in PHBV-microparticles and PLA-nanoparticles. Lat Am J Pharm. 2009;28:393–9.
Melo NFS, Grillo R, Rosa AH, Dias Filho NL, de Paula E, de Araújo DR, et al. Desenvolvimento e caracterização de nanocápsulas de poli(L-lactideo) contendo benzocaína. Quim Nova. 2010;33:65–9.
Kranz H, Bodmeier R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. Eur J Pharm Sci. 2008;34:164–72.
Holgado MA, Arias JL, Cózar MJ, Alvarez-Fuentes J, Ganan-Calvo AM, Fernandez-Arevalo M. Synthesis of lidocaine-loaded PLGA microparticles by flow focusing effects on drug loading and release properties. Int J Pharm. 2008;358:27–35.
Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241–51.
Morales MM. Terapias Avançadas. Rio de Janeiro: Editora Atheneu; 2007.
Losa C, Marchal-Heussler L, Orallo F, Vila Jato JL, Alonso MJ. Design of new formulations for topical ocular administration: Polymeric nanocapsules containing metipranolol. Pharm Res. 1993;10:80–7.
Grillo R, Melo NFS, de Araújo DR, de Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18:688–99.
Fessi H, Puiseiux F, Devissaguet J-P, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:1–4.
Dearden JC, Bresnen GM. The measure of partition coefficients. Quant Struct Act Relat. 1988;7:133–44.
Malheiros SVP, Pinto LMA, Gottardo L, Yokaichiya D, Fraceto LF, Meirelles NC, et al. A new look at hemolytic effect of local anesthetics, considering their real membrane/water partitioning at pH 7,4. Biophys Chem. 2004;110:213–21.
Gamisans F, Lacoulonche F, Chauvet A, Espina M, Garcia ML, Egea MA. Flurbiprofen-loaded nanospheres:analysis of the matrix structure by thermal methods. Int J Pharm. 1999;179:37–48.
Grillo R, Pereira AES, de Melo NFS, Porto RM, Feitosa LO, Tonello PS, et al. Controlled release system for ametryn using polymer microspheres: preparation, characterization and release kinetics in water. J Hazard Mater. 2011;186:1645–51.
Venturini CG, Jager E, Oliveira CP, Bernardi A, Battastini AMO, Guterres SS, et al. Formulation of lipid core nanocapsules. Colloid Surf A Physicochem Eng Asp. 2011;375:200–8.
Paavola A, Yliruusi J, Kajimoto Y, Kalso E, Wahlström T, Rosenberg P. Controlled release of lidocaine from injectable gels and efficacy in rat sciatic nerve block. Pharm Res. 1995;12:1997–2002.
Hariharam D, Peppas NA, Bettini R, Colombo P. Mathematical analysis of drug delivery swellable systems with partial physical restrictions or impermeable coatings. Int J Pharm. 1994;112:47–54.
Colombo P, Bettini R, Massimo G, Catellani PL, Santi P. Peppas NA Drug diffusion front movement is important in drug release control from swellable matrix tablets. J Pharm Sci. 1995;84:991–7.
Ferrero C, Muñoz-Ruiz A, Jiménezcastellanos MR. Fronts movements a useful tool for hydrophilic matrix release mechanism elucidation. Int J Pharm. 2000;202:21–8.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Leszczynska K, Kau ST. A sciatic blockade method to differentiate drug-induced local anesthesia from neuromuscular blockade in mice. J Pharamacol Meth. 1992;27:85–93.
Gantenbein M, Attolini L, Bruguerolle B. Potassium channel agonists modify the local anesthetic activity of bupivacaine in mice. Can J Anaesth. 1996;43:871–6.
Randall LO, Selitto JJ. A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacodyn. 1957;CXI:409–19.
Guterres SS, Fessi H, Barratt G, Devissaguet J-P, Puisieux F. Poly(DL-lactide) nanocapsules containing diclofenac: I. Formulation and stability studies. Int J Pharm. 1995;113:57–63.
Ma J, Feng P, Ye C, Wang Y, Fan Y. An improved interfacial coacervation technique to fabricate biodegradable nanocapsules of an aqueous peptide solution from polylactide and its block copolymers with poly(ethylene glycol). Colloid Polym Sci. 2001;279:387–92.
Stella B, Arpicco S, Rocco F, Marsaud V, Renoir JM, Cattel L, et al. Encapsulation of gemcitabine lipophilic derivatives into polycyanoacrylate nanospheres and nanocapsules. Int J Pharm. 2007;344:71–7.
Fresta M, Cavallaro G, Giammona G, Wehrli E, Puglisi G. Preparation and characterization of polyethyl-2-cyanoacrylate nanocapsules containing antiepileptic drugs. Biomaterials. 1996;17:751–8.
Blouza IL, Charcosset C, Sfarb S, Fessi H. Preparation and characterization of spironolactone-loaded nanocapsules for paediatric use. Int J Pharm. 2006;325:124–31.
Wischke C, Schwendeman SP. Principles of encapsulation of hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364:298–327.
Romero-Cano MS, Vicent B. Controlled release of 4-nitroanisole from poly(lactic acid) nanoparticles. J Control Release. 2002;82:127–35.
Sinnott CJ, Strichartz GR. Levobupivacaine versus ropivacaine for sciatic nerve block in the rat. Reg Anesth Pain Med. 2003;28:294–303.
Ginosar Y, Davidson EM, Firman N, Meroz Y, Lemmens H, Weiniger CF. A randomized controlled trial using patient-controlled epidural analgesia with 0.25% versus 0.0625% bupivacaine in nulliparous labor: effect on analgesia requirement and maternal satisfaction. Int J Obstet Anesth. 2010;19:171–8.
ACKNOWLEDGMENTS
The authors thank FAPESP (processes 06-00121-9 and 07/00127-0), CNPq and Fundunesp for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Melo, N.F.S., Grillo, R., Guilherme, V.A. et al. Poly(Lactide-co-Glycolide) Nanocapsules Containing Benzocaine: Influence of the Composition of the Oily Nucleus on Physico-Chemical Properties and Anesthetic Activity. Pharm Res 28, 1984–1994 (2011). https://doi.org/10.1007/s11095-011-0425-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0425-6