ABSTRACT
Dendrimers have well-organized high branches with a layered architecture providing a series of versatile chemical modification for various purposes. Consequently, this dendrimer nanotechnology explores a new promising class of nanoscale carriers for therapeutic drugs and imaging reagents using passive and active targeting approaches. By controlling dendritic structures, the biological fate of dendrimer/dendrimer-based drugs can be significantly altered based on their intrinsic physicochemical properties, including the hydrophilicity of the unit molecules, particle size, surface charge, and modification. Accordingly, pharmacokinetic aspects play an important role in the design and development of dendrimer systems for successful in vivo application and clinical translation. This review focuses on the recent progress regarding dendritic architectures, structure-related toxicity, and critical factors affecting the pharmacokinetics and biodistribution of dendrimer/dendrimer-based drugs. A better understanding of the basic aspects of dendritic systems and their pharmacokinetics will help to develop a rationale for the design of dendrimers for the controlled delivery of drugs and imaging reagents for therapeutic or diagnostic purposes.




Similar content being viewed by others
REFERENCES
Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60.
Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572–9.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Buhleier E, Wehner W, Vogtle F. “Cascade”-and “Nonskid-Chain-like” syntheses of molecular cavity topologies. Synthesis. 1978;155.
Denkewalter RG, Kolc J, Lukasavage WJ. Macromolecular highly branched homogeneous compound based on lysine units. 1981.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymer: starburst-dendritic macromolecules. Polym J. 1985;17:117–32.
Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. A[27]-arbosol. J Org Chem. 1985;50:2003–4.
Svenson S, Tomalia D. Dendrimers in biomedical applications–reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29.
Wolinsky J, Grinstaff M. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev. 2008;60:1037–55.
Kobayashi H, Brechbiel M. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–86.
Sugao Y, Watanabe K, Higuchi Y, Kurihara R, Kawakami S, Hashida M, et al. NFkappaB decoy delivery using dendritic poly(l-lysine) for treatment of endotoxin-induced hepatitis in mice. Bioorg Med Chem. 2009;17:4990–5.
Dufès C, Uchegbu I, Schätzlein A. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:2177–202.
Hawker C, Fréchet J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–47.
Fréchet J, Hawker C, Gitsov I, Leon JW. Dendrimers and hyperbranched polymers: two families of three-dimensional macromolecules with similar but clearly distinct properties. J Macromol Sci Pure Appl Chem. 1996;33:1399–425.
Lee C, MacKay J, Fréchet J, Szoka F. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26.
Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37.
Cheng Y, Xu T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur J Med Chem. 2008;43:2291–7.
Menjoge A, Kannan R, Tomalia D. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–85.
de Brabander-van den Berg E, Meijer E. Poly(propylene imine) dendrimers: large-scale synthesis by heterogeneously catalyzed hydrogenations. Angew Chem Int Ed Engl. 1993;32:1308–11.
Spetzler J, Tam J. Unprotected peptides as building blocks for branched peptides and peptide dendrimers. Int J Pept Protein Res. 1995;45:78–85.
Fréchet J. Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science. 1994;263:1710–5.
Zhang W, Simanek E. Dendrimers based on melamine. Divergent and orthogonal, convergent syntheses of a G3 dendrimer. Org Lett. 2000;2:843–5.
Wu P, Feldman A, Nugent A, Hawker C, Scheel A, Voit B, et al. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(i)-catalyzed ligation of azides and alkynes. Angew Chem Int Ed Engl. 2004;43:3928–32.
McGrath D. Dendrimer disassembly as a new paradigm for the application of dendritic structures. Mol Pharm. 2005;2:253–63.
Boyd B, Kaminskas L, Karellas P, Krippner G, Lessene R, Porter C. Cationic poly-L-lysine dendrimers: pharmacokinetics, biodistribution, and evidence for metabolism and bioresorption after intravenous administration to rats. Mol Pharm. 2006;3:614–27.
Vega-Villa K, Takemoto J, Yáñez J, Remsberg C, Forrest M, Davies N. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60:929–38.
Roberts J, Bhalgat M, Zera R. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30:53–65.
Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener J, et al. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–48.
Ohsaki M, Okuda T, Wada A, Hirayama T, Niidome T, Aoyagi H. In vitro gene transfection using dendritic poly(L-lysine). Bioconjug Chem. 2002;13:510–7.
Hong S, Bielinska A, Mecke A, Keszler B, Beals J, Shi X, et al. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug Chem. 2004;15:774–82.
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown N, D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–6.
Yang H, Lopina S, DiPersio L, Schmidt S. Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J Mater Sci Mater Med. 2008;19:1991–7.
Bhadra D, Bhadra S, Jain S, Jain N. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–24.
Kolhatkar R, Kitchens K, Swaan P, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug Chem. 2007;18:2054–60.
Asthana A, Chauhan A, Diwan P, Jain N. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech. 2005;6:E536–42.
Kukowska-Latallo J, Candido K, Cao Z, Nigavekar S, Majoros I, Thomas T, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–24.
Lee C, Yoshida M, Fréchet J, Dy E, Szoka F. In vitro and in vivo evaluation of hydrophilic dendronized linear polymers. Bioconjug Chem. 2005;16:535–41.
Gillies E, Dy E, Fréchet J, Szoka F. Biological evaluation of polyester dendrimer: poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. Mol Pharm. 2005;2:129–38.
Miyano T, Wijagkanalan W, Kawakami S, Yamashita F, Hashida M. Anionic amino acid dendrimer-trastuzumab conjugates for specific internalization in HER2-positive cancer cells. Mol Pharm. 2010;7:1318–27.
Neerman M, Zhang W, Parrish A, Simanek E. In vitro and in vivo evaluation of a melamine dendrimer as a vehicle for drug delivery. Int J Pharm. 2004;281:129–32.
Okuda T, Kawakami S, Maeie T, Niidome T, Yamashita F, Hashida M. Biodistribution characteristics of amino acid dendrimers and their PEGylated derivatives after intravenous administration. J Control Release. 2006;114:69–77.
Chen H, Neerman M, Parrish A, Simanek E. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126:10044–8.
Padilla De Jesús O, Ihre H, Gagne L, Fréchet J, Szoka FJ. Polyester dendritic systems for drug delivery applications: in vitro and in vivo evaluation. Bioconjug Chem. 2002;13:453–61.
Tomalia DA, Naylor AM, Goddard III WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl. 1990;29:138–75.
Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug Chem. 2000;11:910–7.
Okuda T, Kawakami S, Akimoto N, Niidome T, Yamashita F, Hashida M. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Control Release. 2006;116:330–6.
Gajbhiye V, Kumar PV, Tekade RK, Jain NK. Pharmaceutical and biomedical potential of PEGylated dendrimers. Curr Pharm Des. 2007;13:415–29.
Shukla S, Wu G, Chatterjee M, Yang W, Sekido M, Diop L, et al. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug Chem. 2003;14:158–67.
Chandrasekar D, Sistla R, Ahmad F, Khar R, Diwan P. The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials. 2007;28:504–12.
Yang W, Barth R, Adams D, Soloway A. Intratumoral delivery of boronated epidermal growth factor for neutron capture therapy of brain tumors. Cancer Res. 1997;57:4333–9.
Shukla R, Thomas T, Peters J, Desai A, Kukowska-Latallo J, Patri A, et al. HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjug Chem. 2006;17:1109–15.
Agashe H, Babbar A, Jain S, Sharma R, Mishra A, Asthana A, et al. Investigations on biodistribution of technetium-99m-labeled carbohydrate-coated poly(propylene imine) dendrimers. Nanomedicine. 2007;3:120–7.
Gurdag S, Khandare J, Stapels S, Matherly L, Kannan R. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug Chem. 2006;17:275–83.
Shukla R, Thomas TP, Desai AM, A. K, Park SJ, Baker JR Jr. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology. 2008;19:art. no. 295102.
Kaminskas L, Kelly B, McLeod V, Boyd B, Krippner G, Williams E, et al. Pharmacokinetics and tumor disposition of PEGylated, methotrexate conjugated poly-l-lysine dendrimers. Mol Pharm. 2009;6:1190–204.
Lee C, Gillies E, Fox M, Guillaudeu S, Fréchet J, Dy E, et al. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci USA. 2006;103:16649–54.
Guillaudeu S, Fox M, Haidar Y, Dy E, Szoka F, Fréchet J. PEGylated dendrimers with core functionality for biological applications. Bioconjug Chem. 2008;19:461–9.
van der Poll D, Kieler-Ferguson H, Floyd W, Guillaudeu S, Jerger K, Szoka F, et al. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconjug Chem. 2010;21:764–73.
Zhu S, Hong M, Zhang L, Tang G, Jiang Y, Pei Y. PEGylated PAMAM dendrimer-doxorubicin conjugates: in vitro evaluation and in vivo tumor accumulation. Pharm Res. 2010;27(1):161–74.
Fox M, Guillaudeu S, Fréchet J, Jerger K, Macaraeg N, Szoka F. Synthesis and in vivo antitumor efficacy of PEGylated poly(l-lysine) dendrimer-camptothecin conjugates. Mol Pharm. 2009;6:1562–72.
Wiener E, Brechbiel M, Brothers H, Magin R, Gansow O, Tomalia D, et al. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;31:1–8.
Konda S, Aref M, Brechbiel M, Wiener E. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000;35:50–7.
Wu G, Barth R, Yang W, Kawabata S, Zhang L, Green-Church K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol Cancer Ther. 2006;5:52–9.
Yang W, Wu G, Barth R, Swindall M, Bandyopadhyaya A, Tjarks W, et al. Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin Cancer Res. 2008;14:883–91.
Perumal O, Inapagolla R, Kannan S, Kannan R. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29:3469–76.
Najlah M, Freeman S, Attwood D, D’Emanuele A. Synthesis, characterization and stability of dendrimer prodrugs. Int J Pharm. 2006;308:175–82.
Kurtoglu Y, Navath R, Wang B, Kannan S, Romero R, Kannan R. Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials. 2009;30:2112–21.
Jansen J, de Brabander-van den Berg E, Meijer E. Encapsulation of guest molecules into a dendritic box. Science. 1994;266:1226–9.
Gupta U, Agashe H, Asthana A, Jain N. Dendrimers: novel polymeric nanoarchitectures for solubility enhancement. Biomacromolecules. 2006;7:649–58.
Cheng Y, Li Y, Wu Q, Zhang J, Xu T. Generation-dependent encapsulation/electrostatic attachment of phenobarbital molecules by poly(amidoamine) dendrimers: evidence from 2D-NOESY investigations. Eur J Med Chem. 2009;44:2219–23.
Ooya T, Lee J, Park K. Effects of ethylene glycol-based graft, star-shaped, and dendritic polymers on solubilization and controlled release of paclitaxel. J Control Release. 2003;93:121–7.
Dhanikula R, Hildgen P. Influence of molecular architecture of polyether-co-polyester dendrimers on the encapsulation and release of methotrexate. Biomaterials. 2007;28:3140–52.
Yang H, Morris J, Lopina S. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J Colloid Interface Sci. 2004;273:148–54.
Dutta T, Jain N. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta. 2007;1770:681–6.
Morgan M, Nakanishi Y, Kroll D, Griset A, Carnahan M, Wathier M, et al. Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 2006;66:11913–21.
Malik N, Evagorou E, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10:767–76.
Chauhan A, Sridevi S, Chalasani K, Jain A, Jain S, Jain N, et al. Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin. J Control Release. 2003;90:335–43.
Patri A, Kukowska-Latallo J, Baker JJ. Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–14.
Kukowska-Latallo J, Bielinska A, Johnson J, Spindler R, Tomalia D, Baker JJ. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci USA. 1996;93:4897–902.
Okuda T, Sugiyama A, Niidome T, Aoyagi H. Characters of dendritic poly(L-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials. 2004;25:537–44.
Radu D, Lai C, Jeftinija K, Rowe E, Jeftinija S, Lin V. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc. 2004;126:13216–7.
Haensler J, Szoka FJ. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993;4:372–9.
Nishikawa M, Takakura Y, Hashida M. Pharmacokinetic evaluation of polymeric carriers. Adv Drug Deliv Rev. 1996;21:135–55.
Takakura Y, Hashida M. Macromolecular carrier systems for targeted drug delivery: pharmacokinetic considerations on biodistribution. Pharm Res. 1996;13:820–31.
Brenner B, Hostetter T, Humes H. Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am J Physiol. 1978;234:F455–60.
Gerlowski L, Jain R. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983;72:1103–27.
Rennke H, Patel Y, Venkatachalam M. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978;13:278–88.
Nishida K, Mihara K, Takino T, Nakane S, Takakura Y, Hashida M, et al. Hepatic disposition characteristics of electrically charged macromolecules in rat in vivo and in the perfused liver. Pharm Res. 1991;8:437–44.
Takakura Y, Fujita T, Furitsu H, Nishikawa M, Sekaki H, Hashida M. Pharmacokinetics of succinylated proteins and dextran sulfate in mice: implications for hepatic targeting of protein drugs by direct succinylation via scavenger receptors. Int J Pharm. 1994;105:19–29.
Dr O, Opsonization PN. biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102.
D’Souza AJ, Topp EM. Release from polymeric prodrugs: linkages and their degradation. J Pharm Sci. 2004;93:1962–79.
Hunt CA, MacGregor RD, Siegel RA. Engineering targeted in vivo drug delivery. I. the physiological and physicochemical principles governing opportunities and limitations. Pharm Res. 1986;3:333–44.
Khandare J, Kolhe P, Pillai O, Kannan S, Lieh-Lai M, Kannan R. Synthesis, cellular transport, and activity of polyamidoamine dendrimer-methylprednisolone conjugates. Bioconjug Chem. 2005;16:330–7.
Khan M, Nigavekar S, Minc L, Kariapper M, Nair B, Lesniak W, et al. In vivo biodistribution of dendrimers and dendrimer nanocomposites – implications for cancer imaging and therapy. Technol Cancer Res Treat. 2005;4:603–13.
Kaminskas L, Boyd B, Karellas P, Henderson S, Giannis M, Krippner G, et al. Impact of surface derivatization of poly-L-lysine dendrimers with anionic arylsulfonate or succinate groups on intravenous pharmacokinetics and disposition. Mol Pharm. 2007;4:949–61.
Wang S, Brechbiel M, Wiener E. Characteristics of a new MRI contrast agent prepared from polypropyleneimine dendrimers, generation 2. Invest Radiol. 2003;38:662–8.
Weiner EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, et al. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;31:1–8.
Kobayashi H, Sato N, Hiraga A, Saga T, Nakamoto Y, Ueda H, et al. 3D-micro-MR angiography of mice using macromolecular MR contrast agents with polyamidoamine dendrimer core with reference to their pharmacokinetic properties. Magn Reson Med. 2001;45:454–60.
Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Konishi J, et al. Micro-MR angiography of normal and intratumoral vessels in mice using dedicated intravascular MR contrast agents with high generation of polyamidoamine dendrimer core: reference to pharmacokinetic properties of dendrimer-based MR contrast agents. J Magn Reson Imaging. 2001;14:705–13.
Bryant LJ, Jordan E, Bulte J, Herynek V, Frank J. Pharmacokinetics of a high-generation dendrimer-Gd-DOTA. Acad Radiol. 2002;9:S29–33.
Bohrer M, Deen W, Robertson C, Troy J, Brenner B. Influence of molecular configuration on the passage of macromolecules across the glomerular capillary wall. J Gen Physiol. 1979;74:583–93.
Sarin H, Kanevsky A, Wu H, Sousa A, Wilson C, Aronova M, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51.
Kobayashi H, Kawamoto S, Choyke P, Sato N, Knopp M, Star R, et al. Comparison of dendrimer-based macromolecular contrast agents for dynamic micro-magnetic resonance lymphangiography. Magn Reson Med. 2003;50:758–66.
Kobayashi H, Kawamoto S, Bernardo M, Brechbiel M, Knopp M, Choyke P. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release. 2006;111:343–51.
Kobayashi H, Kawamoto S, Jo S, Bryant HJ, Brechbiel M, Star R. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem. 2003;14:388–94.
Margerum LD, Campion BK, Koo M, Shargill N, Lai J-J, Marumoto A, et al. Gadolinium(III)DO3A macrocycles and polyethylene glycol coupled to dendrimers: Effect of molecular weight on physical and biological properties of macromolecular magnetic resonance imaging contrast agents. J Alloys Compd. 1997;249:185–90.
Sakharov D, Jie A, Bekkers M, Emeis J, Rijken D. Polylysine as a vehicle for extracellular matrix-targeted local drug delivery, providing high accumulation and long-term retention within the vascular wall. Arterioscler Thromb Vasc Biol. 2001;21:943–8.
Wiwattanapatapee R, Carreño-Gómez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm Res. 2000;17:991–8.
Kitchens K, Kolhatkar R, Swaan P, Ghandehari H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol Pharm. 2008;5:364–9.
Nigavekar S, Sung L, Llanes M, El-Jawahri A, Lawrence T, Becker C, et al. 3H dendrimer nanoparticle organ/tumor distribution. Pharm Res. 2004;21:476–83.
Uehara T, Ishii D, Uemura T, Suzuki H, Kanei T, Takagi K, et al. Gamma-Glutamyl PAMAM dendrimer as versatile precursor for dendrimer-based targeting devices. Bioconjug Chem. 2010;21:175–81.
Kitchens K, Kolhatkar R, Swaan P, Eddington N, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm Res. 2006;23:2818–26.
Parrott M, Benhabbour S, Saab C, Lemon J, Parker S, Valliant J, et al. Synthesis, radiolabeling, and bio-imaging of high-generation polyester dendrimers. J Am Chem Soc. 2009;131:2906–16.
Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, et al. Positive effects of polyethylene glycol conjugation to generation-4 polyamidoamine dendrimers as macromolecular MR contrast agents. Magn Reson Med. 2001;46:781–8.
Zhu S, Hong M, Tang G, Qian L, Lin J, Jiang Y, et al. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: the effects of PEGylation degree and drug conjugation style. Biomaterials. 2010;31:1360–71.
Kaminskas L, Boyd B, Karellas P, Krippner G, Lessene R, Kelly B, et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharm. 2008;5:449–63.
Kaminskas L, Wu Z, Barlow N, Krippner G, Boyd B, Porter C. Partly-PEGylated Poly-L-lysine dendrimers have reduced plasma stability and circulation times compared with fully PEGylated dendrimers. J Pharm Sci. 2009;98:3871–5.
Gillies E, Fréchet J. Designing macromolecules for therapeutic applications: polyester dendrimer-poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. J Am Chem Soc. 2002;124:14137–46.
Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Renal Physiol. 2005;288:F605–13.
Lim J, Guo Y, Rostollan C, Stanfield J, Hsieh J, Sun X, et al. The role of the size and number of polyethylene glycol chains in the biodistribution and tumor localization of triazine dendrimers. Mol Pharm. 2008;5:540–7.
2Hoppe C, Lee Y. The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. Effect of the sugar density. J Biol Chem. 1983;258:14193–9.
Yeeprae W, Kawakami S, Yamashita F, Hashida M. Effect of mannose density on mannose receptor-mediated cellular uptake of mannosylated O/W emulsions by macrophages. J Control Release. 2006;114:193–201.
Wijagkanalan W, Kawakami S, Takenaga M, Igarashi R, Yamashita F, Hashida M. Efficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in rats. J Control Release. 2008;125:121–30.
Barth R, Adams D, Soloway A, Alam F, Darby M. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: evaluation as a potential delivery system for neutron capture therapy. Bioconjug Chem. 1994;5:58–66.
Barth R, Yang W, Adams D, Rotaru J, Shukla S, Sekido M, et al. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res. 2002;62:3159–66.
Yang W, Barth R, Wu G, Kawabata S, Sferra T, Bandyopadhyaya A, et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res. 2006;12:3792–802.
Konda S, Wang S, Brechbiel M, Wiener E. Biodistribution of a 153 Gd-folate dendrimer, generation = 4, in mice with folate-receptor positive and negative ovarian tumor xenografts. Invest Radiol. 2002;37:199–204.
Yao Z, Zhang M, Sakahara H, Saga T, Arano Y, Konishi J. Avidin targeting of intraperitoneal tumor xenografts. J Natl Cancer Inst. 1998;90:25–9.
Wilbur D, Pathare P, Hamlin D, Buhler K, Vessella R. Biotin reagents for antibody pretargeting. 3. Synthesis, radioiodination, and evaluation of biotinylated starburst dendrimers. Bioconjug Chem. 1998;9:813–25.
Kobayashi H, Kawamoto S, Saga T, Sato N, Ishimori T, Konishi J, et al. Avidin-dendrimer-(1B4M-Gd)(254): a tumor-targeting therapeutic agent for gadolinium neutron capture therapy of intraperitoneal disseminated tumor which can be monitored by MRI. Bioconjug Chem. 2001;12:587–93.
Kobayashi H, Kawamoto S, Star R, Waldmann T, Brechbiel M, Choyke P. Activated clearance of a biotinylated macromolecular MRI contrast agent from the blood pool using an avidin chase. Bioconjug Chem. 2003;14:1044–7.
Dijkgraaf I, Rijnders A, Soede A, Dechesne A, van Esse G, Brouwer A, et al. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1, 3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org Biomol Chem. 2007;5:935–44.
Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci USA. 2009;106:685–90.
Huang R, Qu Y, Ke W, Zhu J, Pei Y, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007;21:1117–25.
Mamede M, Saga T, Ishimori T, Higashi T, Sato N, Kobayashi H, et al. Hepatocyte targeting of 111In-labeled oligo-DNA with avidin or avidin-dendrimer complex. J Control Release. 2004;95:133–41.
Kukowska-Latallo J, Raczka E, Quintana A, Chen C, Rymaszewski M, Baker JJ. Intravascular and endobronchial DNA delivery to murine lung tissue using a novel, nonviral vector. Hum Gene Ther. 2000;11:1385–95.
Sato N, Kobayashi H, Saga T, Nakamoto Y, Ishimori T, Togashi K, et al. Tumor targeting and imaging of intraperitoneal tumors by use of antisense oligo-DNA complexed with dendrimers and/or avidin in mice. Clin Cancer Res. 2001;7:3606–12.
Vinter-Jensen L, Frøkiaer J, Jørgensen P, Marqversen J, Rehling M, Dajani E, et al. Tissue distribution of 131I-labelled epidermal growth factor in the pig visualized by dynamic scintigraphy. J Endocrinol. 1995;144:5–12.
Yang W, Barth R, Adams D, Ciesielski M, Fenstermaker R, Shukla S, et al. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res. 2002;62:6552–8.
Bai S, Thomas C, Ahsan F. Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J Pharm Sci. 2007;96:2090–106.
Menjoge A, Navath R, Asad A, Kannan S, Kim C, Romero R, et al. Transport and biodistribution of dendrimers across human fetal membranes: implications for intravaginal administration of dendrimer-drug conjugates. Biomaterials. 2010;31:5007–21.
Wijagkanalan W, Higuchi Y, Kawakami S, Teshima M, Sasaki H, Hashida M. Enhanced anti-inflammation of inhaled dexamethasone palmitate using mannosylated liposomes in an endotoxin-induced lung inflammation model. Mol Pharmacol. 2008;74:1183–92.
Wijagkanalan W, Kawakami S, Higuchi Y, Yamashita F, Hashida M. Intratracheally instilled mannosylated cationic liposome/NFkappaB decoy complexes for effective prevention of LPS-induced lung inflammation. J Control Release. 2010 in press.
McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Tech. 2005;2:89–96.
Kaminskas L, Kota J, McLeod V, Kelly B, Karellas P, Porter C. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J Control Release. 2009;140:108–16.
Florence A, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 2001;50:S69–89.
Florence A, Sakthivel T, Toth I. Oral uptake and translocation of a polylysine dendrimer with a lipid surface. J Control Release. 2000;65:253–9.
Ke W, Zhao Y, Huang R, Jiang C, Pei Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci. 2008;97:2208–16.
Zolnik B, Sadrieh N. Regulatory perspective on the importance of ADME assessment of nanoscale material containing drugs. Adv Drug Deliv Rev. 2009;61:422–7.
Herborn C, Barkhausen J, Paetsch I, Hunold P, Mahler M, Shamsi K, et al. Coronary arteries: contrast-enhanced MR imaging with SH L 643A–experience in 12 volunteers. Radiology. 2003;229:217–23.
McCarthy T, Karellas P, Henderson S, Giannis M, O’Keefe D, Heery G, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2:312–8.
Patton D, Cosgrove Sweeney Y, McCarthy T, Hillier S. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50:1696–700.
Rupp R, Rosenthal S, Stanberry L. VivaGel (SPL7013 Gel): a candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2:561–6.
Yao H, Veine D, Fay K, Staszewski E, Zeng Z, Livant D. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast Cancer Res Treat. 2010 in press.
Kobayashi H, Sato N, Saga T, Nakamoto Y, Ishimori T, Toyama S, et al. Monoclonal antibody-dendrimer conjugates enable radiolabeling of antibody with markedly high specific activity with minimal loss of immunoreactivity. Eur J Nucl Med. 2000;27:1334–9.
Carney P, Rogers P, Johnson D. Dual isotope study of iodine-125 and indium-111-labeled antibody in athymic mice. J Nucl Med. 1989;30:374–84.
Casas A, Battah S, Di Venosa G, Dobbin P, Rodriguez L, Fukuda H, et al. Sustained and efficient porphyrin generation in vivo using dendrimer conjugates of 5-ALA for photodynamic therapy. J Control Release. 2009;135:136–43.
Chauhan A, Jain N, Diwan P, Khopade A. Solubility enhancement of indomethacin with poly(amidoamine) dendrimers and targeting to inflammatory regions of arthritic rats. J Drug Target. 2004;12:575–83.
Chauhan A, Diwan P, Jain N, Tomalia D. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules. 2009;10:1195–202.
Cheng Y, Man N, Xu T, Fu R, Wang X, Wen L. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J Pharm Sci. 2007;96:595–602.
Vandamme T, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102:23–38.
Bhadra D, Bhadra S, Jain N. PEGylated peptide dendrimeric carriers for the delivery of antimalarial drug chloroquine phosphate. Pharm Res. 2006;23:623–33.
Koyama Y, Talanov V, Bernardo M, Hama Y, Regino C, Brechbiel M, et al. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–71.
Subbarayan M, Shetty SJ, Srivastava TS, Noronha OP, Samuel AM, Mukhtar H. Water-soluble 99mTc-labeled dendritic novel porphyrins tumor imaging and diagnosis. Biochem Biophys Res Commun. 2001;281:32–6.
Zhang Y, Sun Y, Xu X, Zhang X, Zhu H, Huang L, et al. Synthesis, biodistribution, and microsingle photon emission computed tomography (SPECT) imaging study of technetium-99m labeled PEGylated dendrimer poly(amidoamine) (PAMAM)-folic acid conjugates. J Med Chem. 2010;53:3262–72.
Sarin H, Kanevsky A, Wu H, Brimacombe K, Fung S, Sousa A, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80.
ACKNOWLEDGMENTS
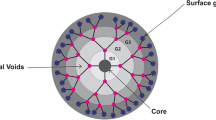
We wish to express sincere thanks to Dr. Yasuhiko Hashida of the iCeMS, Kyoto University, Kyoto, Japan for graphic assistance in Fig. 1a. We are grateful for financial support from W. Wijagkanalan by the Japan Society for the Promotion of Sciences (JSPS) through a JSPS research fellowship for young scientists.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wijagkanalan, W., Kawakami, S. & Hashida, M. Designing Dendrimers for Drug Delivery and Imaging: Pharmacokinetic Considerations. Pharm Res 28, 1500–1519 (2011). https://doi.org/10.1007/s11095-010-0339-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0339-8