Abstract
Purpose
A cell-targeted prodrug with good self-assembly properties in aqueous solution was prepared for the anti-cancer drug paclitaxel, offering great potential for further investigation.
Methods
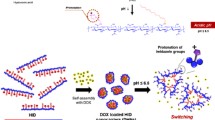
We synthesized hyaluronic acid (HA) with a specific targeting property as a carrier to conjugate with paclitaxel by inserting different amino acids as spacers, including valine, leucine, and phenylalanine, respectively. The structure of HA-amino acid-paclitaxel conjugates was characterized by 1H NMR and GPC. The loading weight and hydrolysis rate were detected by UV and HPLC, respectively. Their morphology and mean diameter were investigated by SEM and DLS, respectively. The biological activity of HA-amino acid-paclitaxel conjugates was measured by MTT assay and flow cytometry using MCF-7 cells.
Results
The use of amino acids as spacers between drug and carrier facilitated paclitaxel release from the conjugates. Their morphology demonstrated that the prepared prodrugs could self-assemble to form nanoparticles with a narrow size distribution and spherical shape. Furthermore, the prodrugs exhibited increased cytotoxicity as compared to free drug. Flow cytometry analysis showed that MCF-7 cells treated with conjugates were arrested in the G2/M phase of the cell cycle.
Conclusions
Prodrugs synthesized as HA-amino acid-paclitaxel conjugates exhibited enhanced cytotoxicity in breast cancer cell lines and hence may have potential application as tumor-specific nanoparticulate therapeutic agents.









Similar content being viewed by others
REFERENCES
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7.
Rowinsky EK, Donehower RC, Jones RJ, Tucker RW. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res. 1988;48:4093–100.
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–8.
Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92.
Francesco MV, Oddone S, Gianfranco P, Raniero M, Ruth D. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate Chem. 2005;16:775–84.
Hsiangfa L, Sungching C, Meichin C, Powei L, Chiungtong C, Hsingwen S. Paclitaxel-loaded poly (γ-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system against cultured HepG2 cells. Bioconjugate Chem. 2006;17:291–9.
Shu-ichi S, Masahiro K, Hiroshi K, To-ru K. Complete regression of xenografted human carcinomas by a paclitaxel-carboxymethyl dextran conjugate (AZ10992). J Control Release. 2007;117:40–50.
Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J Controlled Release. 2000;63:19–30.
Hyukjin L, Kyuri L, Tae PG. Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjugate Chem. 2008;19:1319–25.
Tarr BD, Sambandan TG, Yalkowsky SH. A new parenteral emulsion for the administration of taxol. Pharm Res. 1987;4:162–5.
Bae KH, Lee Y, Park TG. Oil-encapsulating PEO-PPO-PEO shell crosslinked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules. 2007;8:650–6.
Maeda H, Seymour LW, Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjugate Chem. 1992;3:351–62.
Jian Y, Fu-Qiang H, Yong ZD, Hong Y. Polymeric micelles with glycolipid-like structure and multiple hydrophobic domains for mediating molecular target delivery of paclitaxel. Biomacromolecules. 2007;8:2450–6.
Shuliang L, Belinda B, JoEllen W, Andre FP. Self-assembled poly (butadiene)-b-poly (ethylene oxide) polymersomes as paclitaxel carriers. Biotechnol Prog. 2007;23:278–85.
Hyun JL, Hye YN, Byung HL, Dae JK, Jai YK, Jong-sang P. A novel technique for loading of paclitaxel-PLGA nanoparticles onto ePTFE vascular grafts. Biotechnol Prog. 2007;23:693–7.
Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H. Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold. J Controlled Release. 2007;117:380–6.
Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in self-assembled peptide-amphiphile nanofibers. Biomaterials. 2006;27:4079–86.
Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Ectopic bone formation in collagen sponge self-assembled peptide-amphiphile nanofibers hybrid scaffold in a perfusion culture bioreactor. Biomaterials. 2006;27:5089–98.
Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials. 2006;27:5836–44.
Hosseinkhani H, Hosseinkhani M, Kobayashi H. Design of tissue-engineered nanoscaffold through self-assembly of peptide amphiphile. J Bioact Compat Polym. 2006;21:277–96.
Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–77.
Stern R. Association between cancer and “acid mucopolysaccharides”: an old concept comes of age, finally. Semin Cancer Biol. 2008;18:238–43.
Hua Q, Knudson CB, Knudson WJ. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci. 1993;106:365–75.
Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–8.
Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18:244–50.
Coradini D, Pellizzaro C, Miglierini G, Daidone MG, Perbellini A. Hyaluronic acid as drug delivery for sodium butyrate: improvement of the anti-proliferative activity on a breast-cancer cell line. Int J Cancer. 1999;81:411–6.
Coradini D, Zorzet S, Rossin R, Scarlata I, Pellizzaro C, Turrin C, et al. Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res. 2004;10:4822–30.
Speranzaa A, Pellizzaroa C, Coradini D. Hyaluronic acid butyric esters in cancer therapy. Anticancer Drug Des. 2005;16:373–9.
Luo Y, Bernshaw NJ, Lu Z, Kopecek J, Prestwich GD. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm Res. 2002;19:396–402.
Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjugate Chem. 1999;10:755–63.
Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules. 2000;1:208–18.
Wang Y, Xin D, Liu K, Xiang J. Heparin-paclitaxel conjugates using mixed anhydride as intermediate: synthesis, influence of polymer structure on drug release, anticoagulant activity and in vitro efficiency. Pharm Res. 2009;26:785–93.
Coradini D, Pellizzaro C, Abolafio G, Bosco M, Scarlata I, Cantoni S, et al. Hyaluronic-acid butyric esters as promising antineoplastic agents in human lung carcinoma: A preclinical study. Invest New Drug. 2004;22:207–17.
Shu-ichi S, Masahiro K, Hiroshi K, To-ru K. Paclitaxel delivery systems: the use of amino acid linkers in the conjugation of paclitaxel with carboxymethyldextran to create prodrugs. Biol Pharm Bull. 2002;25:632–41.
Peniche C, Arguelles-Monal W, Davidenko N, Sastre R, Gallardo A, Roman J. Self-curing membranes of chitosan/ PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials. 1999;20:1869–78.
Chun L, Dong-fang Y, Robert AN, Fernando CL, Clifton S, Nancy H, et al. Complete regression of well-established tumors using a novel water-soluble Poly(l-Glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58:2404–9.
Rosler A, Vandermeulen GWM, Klok HA. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev. 2001;53:95–108.
Hosseinkhani H, Aoyama T, Yamamoto S, Ogawa O, Tabata Y. In vitro transfection of plasmid DNA by amine derivatives of gelatin accompanied with ultrasound irradiation. Pharm Res. 2002;19:1471–9.
Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Tumor targeting of gene expression through metal-coordinated conjugation with dextran. J Controlled Release. 2003;88:297–312.
Hosseinkhani H, Tabata Y. PEGylation enhances tumor targeting of plasmid DNA by an artificial cationized protein with repeated RGD sequences, Pronectin. J Controlled Release. 2004;97:157–71.
Hosseinkhani H, Azzam T, Tabata Y, Domb AJ. Dextran-spermine polycation: an efficient nonviral vector for in vitro and in vivo gene transfection. Gene Ther. 2004;11:194–203.
Hosseinkhani H, Tabata Y. Ultrasound enhances in vivo tumor expression of plasmid DNA by PEG-introduced cationized dextran. J Controlled Release. 2005;108:540–56.
Nicolaou KC, Rlemer C, Kerr MA, Rideout D, Wrasidlo E. Design, synthesis and biological activity of protaxols. Nature. 1993;364:464–6.
Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci. 1980;77:1561–5.
Jordan MA, Wendll K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–25.
ACKNOWLEDGEMENT
The authors are grateful for the financial support of 985 Foundation of Ministry of Education. This work was supported by the National Natural Science Foundation of China (Grant No. 20472018), by the Natural Science Foundation of Hunan (Key Project No. 07JJ3019), by the Department of Science and Technology of Changsha (Grant No. K082152).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dingcheng Xin and Ying Wang have equal contribution in this work and are both equally considered as first author.
Rights and permissions
About this article
Cite this article
Xin, D., Wang, Y. & Xiang, J. The Use of Amino Acid Linkers in the Conjugation of Paclitaxel with Hyaluronic Acid as Drug Delivery System: Synthesis, Self-Assembled Property, Drug Release, and In Vitro Efficiency. Pharm Res 27, 380–389 (2010). https://doi.org/10.1007/s11095-009-9997-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9997-9