Abstract
Purpose
Labrasol® and Gelucire® 44/14 are defined admixtures of acylglycerols and PEG esters which are substrates for digestive lipases.
Methods
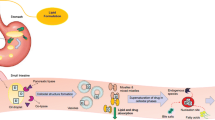
We investigated their in vitro gastrointestinal lipolysis to understand which compounds are, after digestion, responsible for keeping poorly water-soluble drugs in solution. The precipitation of piroxicam and cinnarizine formulated in these excipients during the gastrointestinal lipolysis was also studied.
Results
Monoacylglycerols and PEG monoesters are the largest compounds present at the end of gastric phase whereas PEG-monoesters are the largest compounds after the duodenal phase. The precipitation of piroxicam is mainly due to the gastric lipolysis. In the control experiments performed without digestive lipases, cinnarizine formulated in Labrasol® was found to precipitate upon dilution of the gastric medium to form the solution mimicking the duodenal medium. In the presence of gastric lipase, Labrasol® was hydrolyzed and the precipitation of cinnarizine was not observed in this case. When the cinnarizine was formulated with Gelucire® 44/14 the precipitation was only due to the dilution of the gastric medium.
Conclusion
Our study highlights the importance of the gastrointestinal lipolysis and the associated phenomena such as the dilution of chyme by biliary and pancreatic secretions in vivo, on the solubilisation of poorly water-soluble drugs formulated with lipid-based excipients.



Similar content being viewed by others
REFERENCES
Charman WN, Porter CJH, Mithani S, Dressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and pH. J Pharm Sci. 1997;86:269–82. doi:10.1021/js960085v.
Holm R, Porter CJ, Edwards GA, Müllertz A, Kristensen HG, Charman WN. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci. 2003;20:91–7. doi:10.1016/S0928-0987(03)00174-X.
Khoo SM, Humberstone AJ, Porter CJH, Edwards GA, Charman WN. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int J Pharm. 1998;167:155–64. doi:10.1016/S0378-5173(98)00054-4.
Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235:247–65. doi:10.1016/S0378-5173(02)00003-0.
Vonderscher J, Meinzer A. Rationale for the development of Sandimmune Neoral. Transplant Proc. 1994;26:2925–7.
Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9:87–93. doi:10.1023/A:1018987928936.
Craig DQM, Lievens HSR, Pitt KG, Storey DE. An investigation into the physico-chemical properties of self-emulsifying systems using low frequency dielectric spectroscopy, surface tension measurements and particle size analysis. Int J Pharm. 1993;96:147–55. doi:10.1016/0378-5173(93)90222-2.
Chambin O, Jannin V, Champion D, Chevalier C, Rochat-Gonthier MH, Pourcelot Y. Influence of cryogenic grinding on properties of a self-emulsifying formulation. Int J Pharm. 2004;278:79–89. doi:10.1016/j.ijpharm.2004.02.033.
Barker SA, Yap SP, Yuen KH, McCoy CP, Murphy JR, Craig DQM. An investigation into the structure and bioavailability of α-tocopherol dispersions in Gelucire 44/14. J Control Release. 2003;91:477–88. doi:10.1016/S0168-3659(03)00261-X.
Eaimtrakarn S, Prasad RYV, Ohno T, Konishi T, Yoshikawa Y, Shibata N, et al. Absorption enhancing effect of labrasol on the intestinal absorption of insulin in rats. J Drug Target. 2002;10:255–60. doi:10.1080/10611860290022688.
Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Jayaraj AA, Keirns JJ. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998;87:164–9. doi:10.1021/js970300n.
Hu Z, Riichi T, Takahiro K, Nobuhito S, Kanji T. A novel emulsifier, Labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci. 2001;69:2899–910. doi:10.1016/S0024-3205(01)01375-3.
Hulsmann S, Backensfeld T, Keitel S, Bodmeier R. Melt extrusion—an alternative method for enhancing the dissolution rate of 17-βestradiol hemihydrate. Eur J Pharm Biopharm. 2000;49:237–42. doi:10.1016/S0939-6411(00)00077-1.
Iwanaga K, Kishibiki T, Miyazaki M, Kakemi M. Disposition of lipid-based formulation in the intestinal tract affects the absorption of poorly water-soluble drugs. Biol Pharm Bull. 2006;29:508–12. doi:10.1248/bpb.29.508.
Mehuys E, Remon JP, Korst A, Van Bortel L, Mols R, Augustijns P, et al. Human bioavailability of propranolol from a matrix-in-cylinder system with a HPMC-Gelucire® core. J Control Release. 2005;107:523–36. doi:10.1016/j.jconrel.2005.06.019.
Pillay V, Fassihi R. A new method for dissolution studies of lipid-filled capsules employing nifedipine as a model drug. Pharm Res. 1999;16:333–7. doi:10.1023/A:1011959914706.
Rama Prasad YV, Puthli SP, Eaimtrakarn S, Ishida M, Yoshikawa Y, Shibata N, et al. Enhanced intestinal absorption of vancomycin with Labrasol and D-alpha-tocopheryl PEG 1000 succinate in rats. Int J Pharm. 2003;250:181–90. doi:10.1016/S0378-5173(02)00544-6.
Rama Prasad YV, Minamimoto T, Yoshikawa Y, Shibata N, Mori S, Matsuura A, et al. In situ intestinal absorption studies on low molecular weight heparin in rats using Labrasol as absorption enhancer. Int J Pharm. 2004;271:225–32. doi:10.1016/j.ijpharm.2003.11.013.
Yüksel N, Karatas A, Özkan Y, Savaser A, Özkan SA, Baykara T. Enhanced bioavailability of piroxicam using Gelucire 44/14 and Labrasol: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2003;56:453–9. doi:10.1016/S0939-6411(03)00142-5.
Kossena GA, Boyd BJ, Porter CJ, Charman WN. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J Pharm Sci. 2003;92:634–48. doi:10.1002/jps.10329.
Lindstrom M, Ljusberg-Wahren H, Larsson K, Borgstrom B. Aqueous lipid phases of relevance to intestinal fat digestion and absorption. Lipids. 1981;16:749–54. doi:10.1007/BF02535343.
Fernandez S, Jannin V, Rodier JD, Ritter N, Mahler B, Carriere F. Comparative study on digestive lipase activities on the self emulsifying excipient Labrasol®, medium chain glycerides and PEG esters. Biochim Biophys Acta. 2007;1771:633–40.
Fernandez S, Rodier JD, Ritter N, Mahler B, Demarne F, Carrière F, et al. Lipolysis of the semi-solid self-emulsifying excipient Gelucire® 44/14 by digestive lipases. Biochim Biophys Acta. 2008;1781:367–75.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi:10.1023/A:1016212804288.
Karatas A, Yüksel N, Baykara T. Improved solubility and dissolution rate of piroxicam using gelucire 44/14 and labrasol. Il Farmaco. 2005;60:777–82. doi:10.1016/j.farmac.2005.04.014.
Kossena GA, Charman WN, Boyd BJ, Porter CJH. A novel cubic phase of medium chain lipid origin for the delivery of poorly water soluble drugs. J Control Release. 2004;99:217–29. doi:10.1016/j.jconrel.2004.06.013.
Carriere F, Barrowman JA, Verger R, Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–88.
Lengsfeld H, Beaumier-Gallon G, Chahinian H, De CA, Verger R, Laugier R, et al. Physiology of gastrointestinal lipolysis and therapeutical uses of lipases and digestive lipase inhibitors. In: Müller G, Petry S, editors. Lipases and phospholipases in drug development. Weinheim: Wiley-VCH; 2004. p. 195–229.
Bernback S, Blackberg L, Hernell O. Fatty acids generated by gastric lipase promote human milk triacylglycerol digestion by pancreatic colipase-dependent lipase. Biochim Biophys Acta. 1989;1001:286–93.
Gargouri Y, Pieroni G, Riviere C, Lowe PA, Sauniere JF, Sarda L, et al. Importance of human gastric lipase for intestinal lipolysis : An in vitro study. Biochim Biophys Acta. 1986;879:419–23.
De CJ, Carriere F, Barboni P, Giller T, Verger R, De CA. Pancreatic lipase-related protein 1 (PLRP1) is present in the pancreatic juice of several species. Biochim Biophys Acta. 1998;1387:331–41.
De CJ, Sias B, Grandval P, Ferrato F, Halimi H, Carriere F, et al. Characterization of pancreatic lipase-related protein 2 isolated from human pancreatic juice. Biochim Biophys Acta. 2004;1701:89–99.
Giller T, Buchwald P, Blum-Kaelin D, Hunziker W. Two novel human pancreatic lipase related proteins, hPLRP1 and hPLRP2. Differences in colipase dependence and in lipase activity. J Biol Chem. 1992;267:16509–16.
Eydoux C, De CJ, Ferrato F, Boullanger P, Lafont D, Laugier R, et al. Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells. J Lipid Res. 2007;48:1539–49. doi:10.1194/jlr.M600486-JLR200.
Lombardo D, Fauvel J, Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. I. Action on carboxyl esters, glycerides and phospholipids. Biochim Biophys Acta. 1980;611:136–46.
Sek L, Porter CJ, Charman WN. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situ densitometric analysis. J Pharm Biomed Anal. 2001;25:651–61. doi:10.1016/S0731-7085(00)00528-8.
Sek L, Porter CJ, Kaukonen AM, Charman WN. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J Pharm Pharmacol. 2002;54:29–41. doi:10.1211/0022357021771896.
Carriere F, Moreau H, Gargouri Y, Cudrey C, Ferrato F, Bernadac A, et al. Human gastric lipase. GBF Monograph. 1991;16:129–33.
Carriere F, Bezzine S, Verger R. Molecular evolution of the Pancreatic lipase and two related enzymes towards different substrate selectivities. J Mol Catal B: Enzymatic. 1997;3:55–64. doi:10.1016/S1381-1177(96)00049-5.
Bodmer MW, Angal S, Yarranton GT, Harris TJ, Lyons A, King DJ, et al. Molecular cloning of a human gastric lipase and expression of the enzyme in yeast. Biochim Biophys Acta. 1987;909:237–44.
Vaganay S, Joliff G, Bertaux O, Toselli E, Devignes MD, Benicourt C. The complete cDNA sequence encoding dog gastric lipase. DNA Seq. 1998;8:257–62. doi:10.3109/10425179809008461.
Roussel A, Canaan S, Egloff MP, Riviere M, Dupuis L, Verger R, et al. Crystal structure of human gastric lipase and model of lysosomal acid lipase, two lipolytic enzymes of medical interest. J Biol Chem. 1999;274:16995–7002. doi:10.1074/jbc.274.24.16995.
Roussel A, Miled N, Berti-Dupuis L, Riviere M, Spinelli S, Berna P, et al. Crystal structure of the open form of dog gastric lipase in complex with a phosphonate inhibitor. J Biol Chem. 2002;277:2266–74. doi:10.1074/jbc.M109484200.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi:10.1016/0003-2697(76)90527-3.
Carriere F, Renou C, Lopez V, De CJ, Ferrato F, Lengsfeld H, et al. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterology. 2000;119:949–60. doi:10.1053/gast.2000.18140.
Jinno J, Oh DM, Crison JR, Amidon GL. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J Pharm Sci. 2000;89:268–74. doi:10.1002/(SICI)1520-6017(200002)89:2<268::AID-JPS14>3.0.CO;2-F.
Gu CH, Rao D, Gandhi RB, Hilden J, Raghavan K. Using a novel multicompartment dissolution system to predict the effect of gastric pH on the oral absorption of weak bases with poor intrinsic solubility. J Pharm Sci. 2005;94:199–208. doi:10.1002/jps.20242.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–37. doi:10.1016/j.addr.2007.10.010.
Finholt P, Solvang S. Dissolution kinetics of drugs in human gastric juice—the role of surface tension. J Pharm Sci. 1968;57:1322–6. doi:10.1002/jps.2600570809.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22. doi:10.1023/A:1011984216775.
Galia E, Horton J, Dressman JB. Albendazole generics—a comparative in vitro study. Pharm Res. 1999;16:1871–5. doi:10.1023/A:1018907527253.
Vertzoni M, Pastelli E, Psachoulias D, Kalantzi L, Reppas C. Estimation of intragastric solubility of drugs: in what medium? Pharm Res. 2007;24:909–17. doi:10.1007/s11095-006-9209-9.
Lairon D, Nalbone G, Lafont H, Leonardi J, Domingo N, Hauton JC, et al. Possible roles of bile lipids and colipase in lipase adsorption. Biochem. 1978;17:5263–9. doi:10.1021/bi00617a028.
Fatouros DG, Deen GR, Arleth L, Bergenstahl B, Nielsen FS, Pedersen JS, et al. Structural development of self nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small-angle X-ray scattering. Pharm Res. 2007;24:1844–53. doi:10.1007/s11095-007-9304-6.
Fatouros DG, Bergenstahl B, Mullertz A. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur J Pharm Sci. 2007;31:85–94. doi:10.1016/j.ejps.2007.02.009.
ACKNOWLEDGEMENTS
We are grateful to Cédric Miolane and Christophe Voustinas for their help for the HPLC analysis of piroxicam and cinnarizine, respectively. Sylvie Fernandez’s PhD research was supported by a CIFRE contract from Association Nationale de la Recherche Technique (ANRT, France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandez, S., Chevrier, S., Ritter, N. et al. In Vitro Gastrointestinal Lipolysis of Four Formulations of Piroxicam and Cinnarizine with the Self Emulsifying Excipients Labrasol® and Gelucire® 44/14. Pharm Res 26, 1901–1910 (2009). https://doi.org/10.1007/s11095-009-9906-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9906-2