Abstract
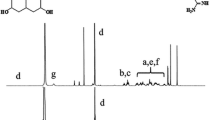
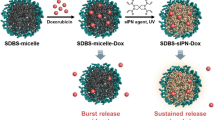
A new pH-sensitive micelle delivery system based on TAT cell penetrating peptide and biodegradable sulfonamide grafted disulfide polymer is presented. The system consists of two components: (1) A polymeric micelle made of Poly(l-lactic acid)-b-poly(ethylene glycol) (PLLA-b-PEG) conjugated to TAT (TAT-micelle), (2) A pH-sensitive diblock copolymer (poly(l-cystine bisamide-g-sulfadiazine))-b-PEG (PCBS-b-PEG). The anionic PCBS complexed with cationic TAT of TAT-micelles forms the final carrier. PCBS showed rapid degradation in the presence of cysteine. The TAT-micelles showed increase in particle size between pH 8.0 and 7.0 upon mixing with PCBS-b-PEG indicating complexation. As the pH was further decreased (pH 6.8 to 6.0) two populations were observed, one of normal TAT-micelles and the other of aggregated PCBS-b-PEG. Flow cytometry showed significantly higher uptake of TAT-micelles at pH 6.6 indicating deshielding compared to pH 7.4. The anticancer drug doxorubicin (DOX) was encapsulated into the TAT-micelles, and the in vitro cytotoxicity at different pHs was evaluated. The system was able to distinguish pHs 7.2 and 7.0 in terms of cytotoxicity.







Similar content being viewed by others
References
E. S. Lee, K. Na, and Y. H. Bae. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 91:103–113 (2003).
S. K. Han, K. Na, and Y. H. Bae. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids Surf., A Physicochem. Eng. Asp. 214:49–59 (2003).
D. E. Discher and A. Eisenberg. Polymer vesicles. Science 297:967–973 (2002).
F. Ahmed and D. E. Discher. Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles. J. Control. Release 96:37–53 (2004).
I. Bala, S. Hariharan, and M. N. Kumar. PLGA nanoparticles in drug delivery: the state of the art. Crit. Rev. Ther. Drug Carr. Syst. 21:387–422 (2004).
J. Panyam and V. Labhasetwar. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55:329–347 (2003).
M. Yokoyama, G. S. Kwon, T. Okano, Y. Sakurai, T. Seto, and K. Kataoka. Preparation of micelle-forming polymer-drug conjugates. Bioconjug. Chem. 3:295–301 (1992).
K. Na, V. A. Sethuraman, and Y. H. Bae. Stimuli-sensitive polymeric micelles as anticancer drug carriers. Anticancer Agents Med. Chem. 6:525–535 (2006).
S. R. Croy and G. S. Kwon. Polymeric micelles for drug delivery. Curr. Pharm. Des. 12:4669–4684 (2006).
R. Kim. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 103:1551–1560 (2005).
N. R. Wardwell and P. P. Massion. Novel strategies for the early detection and prevention of lung cancer. Semin. Oncol. 32:259–268 (2005).
T. Minko, S. S. Dharap, R. I. Pakunlu, and Y. Wang. Molecular targeting of drug delivery systems to cancer. Current Drug Targets 5:389–406 (2004).
M. Richter and H. Zhang. Receptor-targeted cancer therapy. DNA Cell Biol. 24:271–282 (2005).
C. I. Spiridon, S. Guinn, and E. S. Vitetta. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin. Cancer Res. 10:3542–3551 (2004).
S. P. Vyas, A. Singh, and V. Sihorkar. Ligand–receptor-mediated drug delivery: an emerging paradigm in cellular drug targeting. Crit. Rev. Ther. Drug Carr. Syst. 18:1–76 (2001).
V. A. Sethuraman and Y. H. Bae. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release 118:216–224 (2006).
R. J. Christie and D. W. Grainger. Design strategies to improve soluble macromolecular delivery constructs. Adv. Drug Deliv. Rev. 55:421–437 (2003).
M. Meyer and E. Wagner. pH-responsive shielding of non-viral gene vectors. Exp. Opin. Drug Deliv. 3:563–571 (2006).
A. Nori, K. D. Jensen, M. Tijerina, P. Kopeckova, and J. Kopecek. Subcellular trafficking of HPMA copolymer-Tat conjugates in human ovarian carcinoma cells. J. Control. Release 91:53–59 (2003).
L. Hyndman, J. L. Lemoine, L. Huang, D. J. Porteous, A. C. Boyd, and X. Nan. HIV-1 Tat protein transduction domain peptide facilitates gene transfer in combination with cationic liposomes. J. Control. Release 99:435–444 (2004).
S. Pujals, J. Fernandez-Carneado, C. Lopez-Iglesias, M. J. Kogan, and E. Giralt. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim. Biophys. Acta 1758:264–279 (2006).
A. S. Ojugo, P. M. McSheehy, D. J. McIntyre, C. McCoy, M. Stubbs, M. O. Leach, I. R. Judson, and J. R. Griffiths. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 12:495–504 (1999).
M. Stubbs, P. M. McSheehy, J. R. Griffiths, and C. L. Bashford. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 6:15–19 (2000).
W. Yu, K. F. Pirollo, A. Rait, B. Yu, L. M. Xiang, W. Q. Huang, Q. Zhou, G. Ertem, and E. H. Chang. A sterically stabilized immunolipoplex for systemic administration of a therapeutic gene. Gene. Ther. 11:1434–1440 (2004).
G. M. Kim, Y. H. Bae, and W. H. Jo. pH-induced micelle formation of poly(histidine-co-phenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromol. Biosci. 5:1118–1124 (2005).
S. I. Kang and Y. H. Bae. pH-induced volume-phase transition of hydrogels containing sulfonamide side group by reversible crystal formation. Macromolecules 34:8173–8178 (2001).
S. I. Kang and Y. H. Bae. pH-induced solubility transition of sulfonamide-based polymers. J. Control. Release 80:145–155 (2002).
S. I. Kang, K. Na, and Y. H. Bae. Sulfonamide-containing polymers: a new class of pH-sensitive polymers and gels. Macromol. Symp. 172:149–156 (2001).
Y. Zong, X. Wang, K. C. Goodrich, A. M. Mohs, D. L. Parker, and Z. R. Lu. Contrast-enhanced MRI with new biodegradable macromolecular Gd(III) complexes in tumor-bearing mice. Magn. Reson. Med. 53:835–842 (2005).
A. Lucke, J. Tessmar, E. Schnell, G. Schmeer, and A. Gopferich. Biodegradable poly(D,L-lactic acid)-poly(ethylene glycol)-monomethyl ether diblock copolymers: structures and surface properties relevant to their use as biomaterials. Biomaterials 21:2361–2370 (2000).
K. Kataoka, A. Harada, and Y. Nagasaki. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 47:113–131 (2001).
V. A. Sethuraman and Y. H. Bae. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release 118:216–224 (2007).
T. L. Kaneshiro, T. Ke, E. K. Jeong, D. L. Parker, and Z. R. Lu. Gd-DTPA L-cystine bisamide copolymers as novel biodegradable macromolecular contrast agents for MR blood pool imaging. Pharm. Res. 23:1285–1294 (2006).
V. A. Sethuraman, K. Na, and Y. H. Bae. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: an in vitro study. Biomacromolecules 7:64–70 (2006).
S. I. Kang and Y. H. Bae. A sulfonamide based glucose-responsive hydrogel with covalently immobilized glucose oxidase and catalase. J. Control. Release 86:115–121 (2003).
D. C. Bibby, N. M. Davies, and I. G. Tucker. Poly(acrylic acid) microspheres containing beta-cyclodextrin: loading and in vitro release of two dyes. Int. J. Pharm. 187:243–250 (1999).
C. Ramkissoon-Ganorkar, F. Liu, M. Baudys, and S. W. Kim. Modulating insulin-release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J. Control. Release 59:287–298 (1999).
N. Raghunand, R. A. Gatenby, and R. J. Gillies. Microenvironmental and cellular consequences of altered blood flow in tumours. Br. J. Radiol. 76(Spec No 1):S11-S22 (2003).
X. L. Yang and A. H. Wang. Structural studies of atom-specific anticancer drugs acting on DNA. Pharmacol. Ther. 83:181–215 (1999).
Acknowledgments
The authors wish to thank the Core Facilities at the University of Utah for use of the Mass and NMR spectroscopy and flow cytometer. This work was supported by NIH CA122356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sethuraman, V.A., Lee, M.C. & Bae, Y.H. A Biodegradable pH-sensitive Micelle System for Targeting Acidic Solid Tumors. Pharm Res 25, 657–666 (2008). https://doi.org/10.1007/s11095-007-9480-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9480-4